| Complex | charge | specific conductance (ohm-1 cm2) |
|---|---|---|
| [Co(NH3)6]Cl3 | 3 | 431.6 |
| [Co(NH3)6]Br3 | 3 | 426.9 |
| [Co(NH3)6](NO2)3 | 3 | 421.9 |
| [Co(NH3)5(H2O)]Br3 | 3 | 412.9 |
| [Co(NH3)4(H2O)2]Br3 | 3 | 399.5 |
| [Co(NH3)3(H2O)3]Br 3 | 3 | 383.8 |
| [Co(NH3)5Cl]Cl2 | 2 | 261.4 |
| [Co(NH3)5(ONO)]Cl2 | 2 | 258.4 |
| [Co(NH3)5Br]Br2 | 2 | 257.6 |
| [Co(NH3)5(NO2)]Cl2 | 2 | 246.4 |
| [Co(NH3)5(NO2)]Br2 | 2 | 236.8 |
| [Co(NH3)5(NO2)]( NO2)2 | 2 | 234.4 |
| [Co(NH3)4(CO3)]Br | 1 | 106 |
| cis-[Co(NH3)4(NO2)2]Cl | 1 | 100.7 |
| trans-[Co(NH3)4(NO2)2]Cl | 1 | 98.35 |
| [Co(NH3)3(NO2)3] | 0 | <0 |
| K[Co(NH3)2(NO2)4] | -1 | 99.29 |
| K2[Co(NH3)( NO2)5] - unknown | -2 | ? |
| K3[Co(NO2)6] ** | -3 | ~560 |
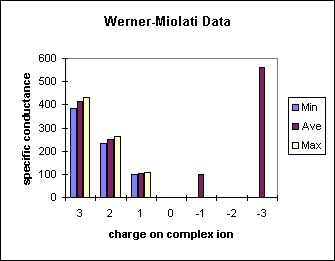
| Charge | Specific Conductance (ohm-1 cm2) | ||
|---|---|---|---|
| Min | Ave | Max | |
| 3 | 383.8 | 412.8 | 431.6 |
| 2 | 234.4 | 249.2 | 261.4 |
| 1 | 98.35 | 101.7 | 106 |
| 0 | <10 | ||
| -1 | 99.29 | ||
| -2 | ? | ||
| -3 | ~560 | ||
A gap exists in the chart for a dianionic cobalt complex.
A few complexes of this type have been prepared since then, such
as K2[Co(NH3)CN5] and
[Co(NH3)C2O4(NO2)
3]2-. What information can you find about these
complexes? Have their specific conductances been measured?
Why not suggest a few others that could be used instead? The sort
of complex you would need is
[Co(NH3)(C2O4)2(NO
2)]2-. What others can you think of?
 Return to Chemistry,
UWI-Mona, Home Page
Return to Chemistry,
UWI-Mona, Home Page
Copyright © 2005 by Robert John Lancashire, all rights reserved.
Created and maintained by Prof. Robert J. Lancashire,