Spectroscopy of First Row Transition Metal Complexes
Crystal Field Theory copes reasonably well
for d1 (d9) systems but not for
multi-electron systems, which are the more common. To deal with
these systems we need to introduce a new concept, that of the
electronic state.
Electronic configurations refer to the way in which the electrons
occupy the d orbitals, so for Ti(III) we write an electronic
configuration of [Ar] 3d1 and in an octahedral crystal
field the lowest energy configuration would be written as
t2g1 eg0.
The electronic state refers to energy levels available to a group
of electrons. This is much more complex than the single electron
case since not only is it necessary to consider the crystal field
effects of the repulsion of the metal electrons by the ligand
electrons, but it is necessary to include the interactions between
the electrons themselves.
When describing electronic configurations, lower case letters are
used, thus t2g1 etc.
For electronic states, upper case (CAPITAL) letters are used and
by analogy, a T state is triply degenerate. Subscripts 1 and 2
are used to distinguish states of like degeneracy and g and u
subscripts indicate the presence of a centre of symmetry eg.
T1g, T2g, T1u and
T2u.
These symbols are further modified to show the spin multiplicity
of the electronic state using the Russell-Saunders Notation.
If we consider the Ti(III) case, the electronic configuration is
d1. In an octahedral crystal field this would give
rise to a t2g1 arrangement and the
excitation of the low lying electron to the higher level would
then give an eg1 arrangement.
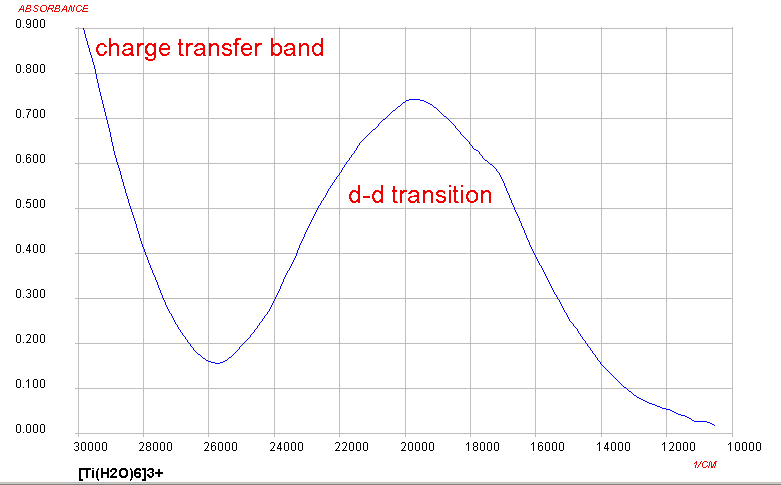
Only one d-d transition is expected and this roughly corresponds to
what is observed for Ti(III) complexes, although it is somewhat
more complicated due to Jahn-Teller
considerations. At the high energy end of the spectrum, the
presence of a charge transfer band should be noted as well. The origin
of this will not be covered in detail in this course.
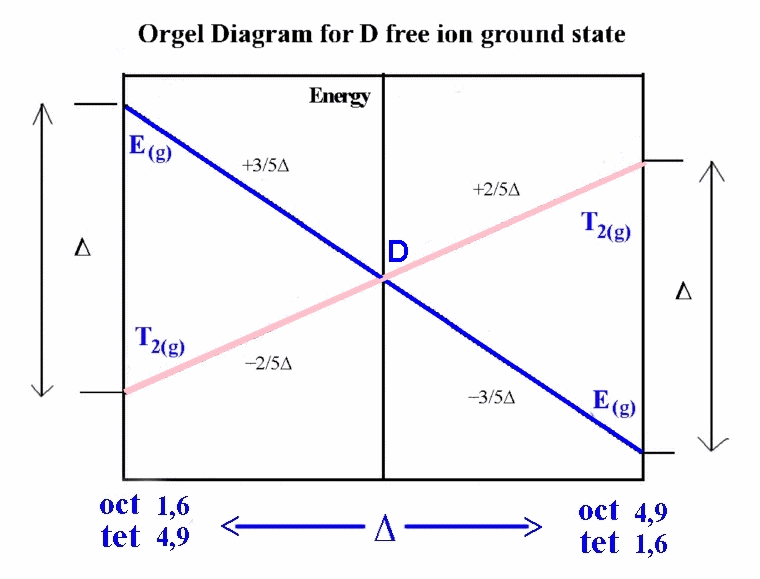
One approach taken when we consider the Russell-Saunders scheme
with the various electronic states makes use of what are called
Orgel diagrams. The relevant Orgel diagram for the D ground state
is given below:
 oct 4,9 tet 1,6 <-------------------------------------------> oct 1,6 tet 4,9
oct 4,9 tet 1,6 <-------------------------------------------> oct 1,6 tet 4,9
For a Fe(II) high spin octahedral complex we would write the free
ion electronic configuration as d6 and in the
octahedral crystal field it would be described as
t2g4 eg2.
The Russell-Saunders scheme that takes into account the
electron-electron interactions would be described by a free ion
ground state of 5D. In octahedral and tetrahedral
crystal fields, this D state is split into E(g) and
T2(g) terms. To decide which side of the D Orgel
diagram should be applied to the interpretation can be quickly
determined by looking at the electronic configuration and noting
that the ground state is triply degenerate and the excited state is doubly degenerate
(i.e. we must use the right-hand-side).
It is expected then that there should be 1 absorption band found
in the electronic spectrum and that the energy of the transition
corresponds directly to Δ. The transition is written in a
notation that is read from right to left, which in this case is
5Eg ← 5T2g.
Continue on to free ion F states.
return to the CHEM2101 (C21J) course
outline
 Return to Chemistry, UWI-Mona,
Home Page
Return to Chemistry, UWI-Mona,
Home Page
Copyright © 2005-2010 by Robert John
Lancashire, all rights reserved.
Created and maintained by Prof. Robert J.
Lancashire
The Department of Chemistry, University of the West Indies,
Mona Campus, Kingston 7, Jamaica.
Created September 2005. Links checked and/or
last modified 20th September 2010.
URL
http://wwwchem.uwimona.edu.jm/courses/C21JSpec_D.html


 Return to Chemistry, UWI-Mona,
Home Page
Return to Chemistry, UWI-Mona,
Home Page