Unit - Chemistry of Garments: Leather
While an animal is alive, its skin is soft, flexible, very tough
and hard wearing and essentially semipermeable: meaning that
although water vapour can travel out, it is not able to penetrate
and come in. This changes when the animal dies. If the skin is
then kept moist it deteriorates by rotting, and if it is dried it
goes hard and brittle.
The purpose of the tanning
process is stabilise its structure so that the skin (from animals including
goats and kangaroos, but more often cattle hide) can retain it's natural
properties. After chemically processing the skin, it will no longer be
subject to putrefecation.
Tanning is essentially the reaction of collagen fibers in the
hide with tannins, chromium, alum, or other chemical agents. The
most common tanning agents used in the U.S. are trivalent
chromium and vegetable tannins extracted from specific tree
barks. Alum, syntans (man-made chemicals), formaldehyde,
glutaraldehyde, and heavy oils are other tanning agents.
There are approximately 111 leather tanning facilities in the
United States. However, not every facility may perform the entire
tanning or finishing process. Leather tanning and finishing
facilities are most prevalent in the northeast and midwest
states; Pennsylvania, Massachusetts, New York, and Wisconsin
account for almost half of the facilities. The number of
tanneries in the United States has significantly decreased in the
last 40 years due to the development of synthetic substitutes for
leather, increased leather imports, and environmental
regulations.
Traditionally, tanning used tannins, bitter plant
polyphenolic compounds that bind to and precipitate proteins and
various other organic compounds including amino acids and
alkaloids.
The term tannin (from tanna, an Old High German word for oak or
fir tree, as in Tannenbaum) refers to the use of wood tannins
from oak in tanning animal hides into leather; hence the words
"tan" and "tanning" for the treatment of leather. However, the
term "tannin" by extension is widely applied to any large
polyphenolic compound containing sufficient hydroxyls and other
suitable groups (such as carboxyls) to form strong complexes with
proteins and other macromolecules.
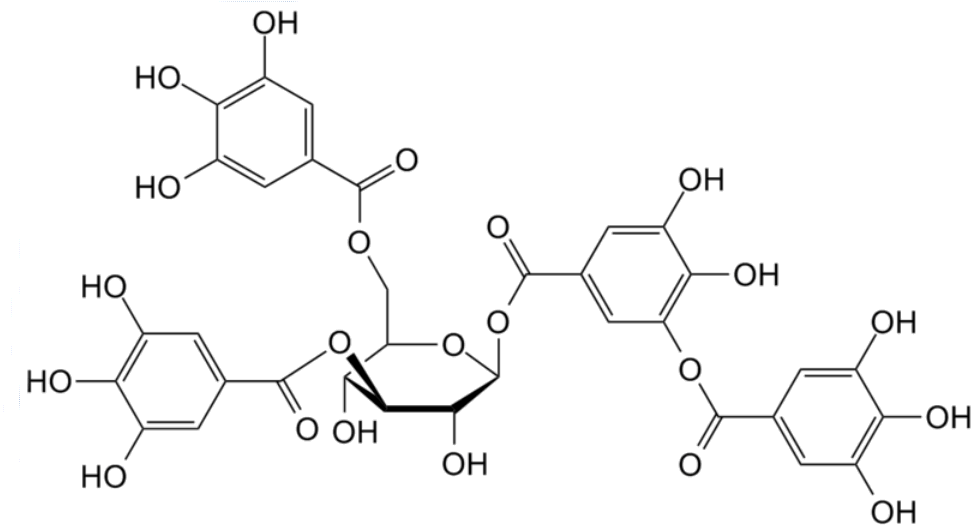
|
|
| The structure of some (gallo)tannic acids |
Leather is a
durable and flexible material and can be produced through
manufacturing processes that range from cottage to heavy
industries.
The processing can be divided into three sub-processes: the
preparatory stage, tanning and crusting. All true leathers will
undergo these sub-processes. A further sub-process, surface
coating may be added into the sequence. The list of operations
that leathers undergo varies with the type of leather required,
but can be roughly described by the following eight steps:
Step 1 - Unhairing
The animal skins are steeped in an alkali solution that breaks
down the structure of the hair at its weakest point (the root)
and so removes the hair.
Step 2 - Liming
The hairless skin is immersed in a solution of alkali and sulfide
to complete the removal of the hair and to alter the properties
of the skin protein (collagen). The collagen becomes chemically
modified and swells, leaving a more open structure.
Step 3 - Deliming and Bating
The skin structure is then opened further by treatment with
enzymes, and further unwanted material is removed.
Step 4 - Pickling
The skins are then treated with acid to preserve them for up to
two years
Step 5 - Tanning
This is the most chemically complex step. During tanning, the
skin structure is stabilised in its open form by replacing some
of the collagen with complex ions of chromium. Depending on the
compounds used, the colour and texture of the leather changes.
When leather has been tanned it is able to 'breathe' and to
withstand 100 °C boiling water, as well as being much more
flexible than an untreated dead skin.
Step 6 - Neutralising, Dyeing and Fat
Liquoring
The leather is then treated with alkali to neutralise it and so
prevent deterioration, and then dyed. This involves fixing a
variety of compounds onto the chromium, as that is the most
reactive site present. Once the leather is dyed, it is treated
with reactive oils that attach themselves to the fibrous
structure, improving suppleness and flexibility.
Step 7 - Drying
Water is removed from the leather, and its chemical properties
stabilised.
Step 8 - Finishing
A surface coating is applied to ensure an even colour and
texture, and to improve its ability to wear. Suede leather is
also buffed at this point to give it its distinctive
finish.
Heavy leathers and sole leathers are produced by the vegetable
tanning process, the oldest of any process in use in the leather
tanning industry.
In the vegetable tanning process, the concentration of the
tanning materials starts out low and is gradually increased as
the tannage proceeds. It usually takes 3 weeks for the tanning
material to penetrate to the center of the hide. The skins or
hides are then wrung and may be cropped or split; heavy hides may
be retanned and scrubbed. For sole leather, the hides are
commonly dipped in vats or drums containing sodium bicarbonate or
sulfuric acid for bleaching and removal of surface tannins.
Materials such as lignosulfate, corn sugar, oils, and specialty
chemicals may be added to the leather. The leather is then set
out to smooth and dry and may then undergo further finishing
steps. However, a high percentage of vegetable-tanned leathers do
not undergo retanning, coloring, fatliquoring, or
finishing.
Leather may be dried by any of five common methods. Air drying is
the simplest method. The leather is hung or placed on racks and
dried by the natural circulation of air around it. A toggling
unit consists of a number of screens placed in a dryer that has
controlled temperature and humidity. In a pasting unit, leathers
are pasted on large sheets of plate glass, porcelain, or metal
and sent through a tunnel dryer with several controlled
temperature and humidity zones. In vacuum drying, the leather is
spread out, grain down, on a smooth surface to which heat is
applied. A vacuum hood is placed over the surface, and a vacuum
is applied to aid in drying the leather. High-frequency drying
involves the use of a high frequency electromagnetic field to dry
the leather.
Chrome-tanned leather tends to be softer and more pliable than
vegetable-tanned leather, has higher thermal stability, is very
stable in water, and takes less time to produce than
vegetable-tanned leather. Almost all leather made from
lighter-weight cattle hides and from the skin of sheep, lambs,
goats, and pigs is chrome tanned. The first steps of the process
(soaking, fleshing, liming/dehairing, deliming, bating, and
pickling) and the drying/finishing steps are essentially the same
as in vegetable tanning. However, in chrome tanning, the
additional processes of retanning, dyeing, and fatliquoring are
usually performed to produce usable leathers and a preliminary
degreasing step may be necessary when using animal skins, such as
sheepskin.
Leather that is not subject to scuffs and scratches need only
be surface dyed. For other types of leather (e.g., shoe leather)
the dye must penetrate further into the leather. Typical
dyestuffs are aniline-based compounds that combine with the skin
to form an insoluble compound.
Leather Finishing
Leathers may be finished in a variety of ways: buffed with fine
abrasives to produce a suede finish; waxed, shellacked, or
treated with pigments, dyes, and resins to achieve a smooth,
polished surface and the desired colour; or lacquered with
urethane for a glossy patent leather. Water-based or
solvent-based finishes may then be applied to the leather.
Plating is used to smooth the surface of the coating
materials and bond them to the grain. Hides may be embossed.
Environmental Issues
1 metric ton of raw hide yields 250-300 kg of leather but also leaves
600 kg of solid waste, including sludge.
The presence of Cr(VI) in leather and leather products is a cause
of concern. Cr(VI) is bioaccumulating, highly toxic, mutagenic
and carcinogenic to humans. Cr(VI) is not used at any stage in
the manufacturing process, only Cr(III) which is not
carcinogenic. Where does the oxidation occur and how it can be
avoided are critical questions for chemists working in the
leather industry.
Shoe-Polish
See the YouTube video for how
Kiwi shoe polish is made.
Prior to 1906, shoe polish was not well known as a commercial product,
nor was it particularly sophisticated. While sales were not especially high,
a few brands existed, like Nugget, during the 19th century. The practice of shining shoes
gradually caught on and soon shoeshine boys in city streets were offering shoe
shines using a basic form of shoe polish along with a polishing cloth.
Shoe polish (or boot polish), is a waxy paste, cream, or liquid used to
polish, shine, and waterproof leather shoes or boots to extend the
footwear's life, and restore, maintain and improve their appearance.
Various substances have been used as shoe polish for hundreds of years,
starting with natural substances such as wax and tallow. Modern polish
formulas were introduced early in the 20th century and many of those
original formulations are still in use today. Today, shoe polish is
usually made from a mix of natural and synthetic materials, including
naphtha, turpentine, dyes, and gum arabic, using straightforward chemical
engineering processes. Shoe polish is usually flammable, can be toxic,
and, if misused, can stain skin.
The first shoe polish to resemble the modern varieties (aimed primarily at
inducing shine) was Kiwi. Scottish expatriates William Ramsay and
Hamilton McKellan began making "boot polish" in a small factory in 1904
in Melbourne, Australia. Their formula was a major improvement on previous
brands. It preserved shoe leather, made it shine, and restored color.
By the time Kiwi Dark Tan was released in 1908, it incorporated agents that
added suppleness and water resistance. Australian-made boot polish was then
considered the world's best. Black and a range of colors became available,
and exports to Britain, continental Europe, and New Zealand began.
Shoe polish consists of a waxy colloidal emulsion, a substance composed of a
number of partially immiscible liquids and solids mixed together. It is
usually made from ingredients including some or all of naphtha, lanolin,
turpentine, wax
(often Carnauba wax),
gum arabic, ethylene glycol,
and if required a colourant, such as carbon black or an azo dye
(such as aniline yellow). It typically has a specific gravity of 0.8,
is negligibly soluble in water, and is made of between 65 and 77%
volatile substances - usually naphtha.
Carnauba wax consists mostly of aliphatic esters (40 wt%), diesters of
4-hydroxycinnamic acid (a.k.a.
p-coumaric acid
21.0 wt%),
ω-hydroxycarboxylic acids (13.0 wt%) and fatty acid alcohols (12 wt%).
The high amount of volatile substances means that the shoe polish will dry out
and harden after application, while retaining its shine.
References
New Zealand Institute of Chemistry (NZIC) article on Leather
processing
FAO report
EPA report on Leather Tanning
Acknowledgements.
Much of the information in these course notes has been sourced
from Wikipedia under the Creative Commons License. Students
taking this course will be expected to contribute to Wikipedia as
a part of their course assignments.
return to CHEM2402 course
outline.

This work is licensed under a Creative Commons
Attribution-ShareAlike 3.0 Unported License.
 Return to Chemistry,
UWI-Mona, Home Page
Created and maintained by Prof. Robert J.
Lancashire,
Return to Chemistry,
UWI-Mona, Home Page
Created and maintained by Prof. Robert J.
Lancashire,
The Department of Chemistry, University of the West Indies,
Mona Campus, Kingston 7, Jamaica
Created February 2013. Links checked and/or last
modified 12th October 2016.
URL
http://wwwchem.uwimona.edu.jm/courses/CHEM2402/Textiles/Leather.html



 Return to Chemistry,
UWI-Mona, Home Page
Created and maintained by Prof. Robert J.
Lancashire,
Return to Chemistry,
UWI-Mona, Home Page
Created and maintained by Prof. Robert J.
Lancashire,