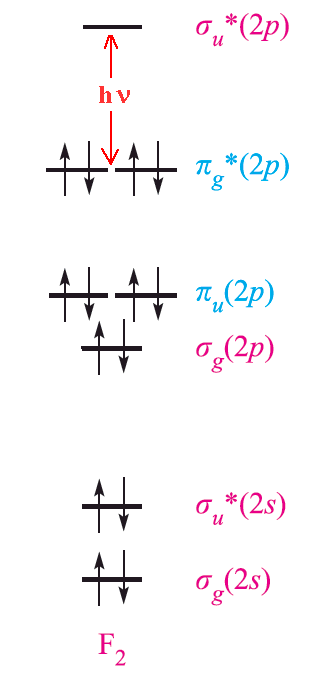
Some chemical and physical properties of the halogens are
summarized in the Table below.
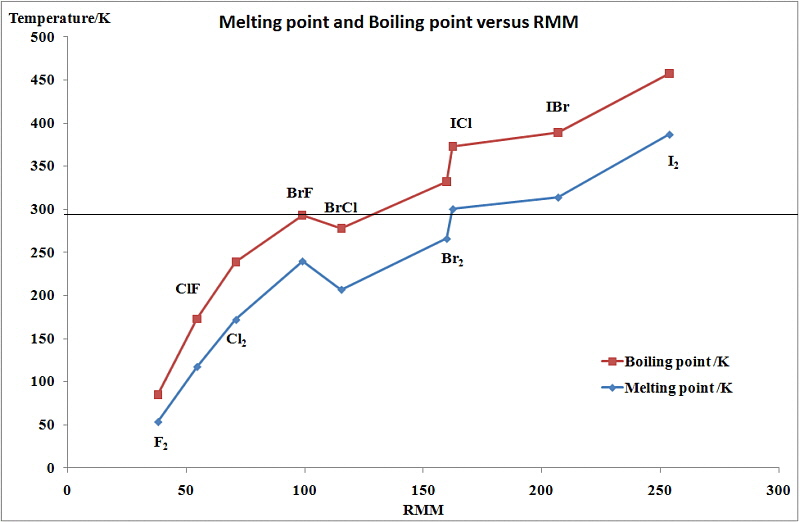
It can be seen that there is a regular increase in many of the
properties of the halogens proceeding down group 17 from fluorine
to iodine. This includes their melting points, boiling points,
intensity of their colour, the radius of the corresponding halide
ion, and the density of the element. On the other hand, there is
a regular decrease in the first ionization energy as we go down
this group. As a result, there is a regular increase in the
ability to form high oxidation states and a decrease in the
oxidizing strength of the halogens from fluorine to iodine.
| Properties of Group 17 (The Halogens) | F | Cl | Br | I |
|---|---|---|---|---|
| Atomic number, Z | 9 | 17 | 35 | 53 |
| Ground state electronic configuration | [He]2s2 2p5 | [Ne]3s2 3p5 | [Ar]3d10 4s2 4p5 | [Kr]4d10 5s2 5p5 |
| Colour | pale yellow gas | yellow-green gas | red-brown liquid | blue-black solid |
| Density of liquids at various temperatures, /kg m-3 | 1.51 (85 °K) | 1.66 (203 °K) | 3.19 (273 °K) | 3.96 (393 °K) |
| Melting point, /K | 53.53 | 171.6 | 265.8 | 386.85 |
| Boiling point, /K | 85.01 | 239.18 | 331.93 | 457.5 |
| Enthalpy of atomisation, ΔaH° (298K) / kJ mol-1 | 79.08 | 121.8 | 111.7 | 106.7 |
| Standard enthalpy of fusion of X2, ΔfusH°(mp) / kJ mol-1 | 0.51 | 6.4 | 10.57 | 15.52 |
| Standard enthalpy of vaporization of X2, ΔvapH°(bp) / kJ mol-1 | 6.62 | 20.41 | 29.96 | 41.57 |
| First ionization energy, IE1 / kJ mol-1 | 1681 | 1251.1 | 1139.9 | 1008.4 |
| ΔEAH1°(298K) / kJ mol-1 | -333 | -348 | -324 | -295 |
| ΔhydH°(X-,g) / kJ mol-1 | -504 | -361 | -330 | -285 |
| ΔhydS°(X-,g) / JK-1 mol-1 | -150 | -90 | -70 | -50 |
| ΔhydG°(X-,g) / kJ mol-1 | -459 | -334 | -309 | -270 |
| Standard redox potential, E°(X2 /2X-) /V | 2.87 | 1.36 | 1.09 | 0.54 |
| Covalent radius, rcov = ½ X-X bond length /pm | 72 | 100 | 114.2 | 133.3 |
| Ionic radius, rion for X- /pm | 133 | 181 | 196 | 220 |
| van der Waals radius, rv /pm | 135 | 180 | 195 | 215 |
| X-X(g)bond energy /kJ mol-1 | 159 | 243 | 193 | 151 |
| H-X(g)bond energy /kJ mol-1 | 562 | 431 | 366 | 299 |
| C-X(g)bond energy /kJ mol-1 | 484 | 338 | 276 | 238 |
| Pauling electronegativity, χP | 3.98 | 3.16 | 2.96 | 2.66 |
The amount of energy required for excitation depends upon the size of the atom. Fluorine is the smallest element in the group and the force of attraction between the nucleus and the outer electrons is very large. As a result, it requires a large excitation energy and absorbs violet light (high energy) and so appears pale yellow. On the other hand, iodine needs significantly less excitation energy and absorbs yellow light of low energy. Thus it appears dark violet. Using similar arguments, it is possible to explain the greenish yellow color of chlorine and the reddish brown color of bromine.
The halogens show a variety of colours when dissolved in different solvents. Solutions of iodine can be bright violet in CCl4, pink or reddish brown in aromatic hydrocarbons and deep brown in alcohols for example. This can be explained by weak donor-acceptor interaction and complex formation. The presence of charge-transfer bands further supports this since they are thought to be derived from interaction with the HOMO σu* orbital.
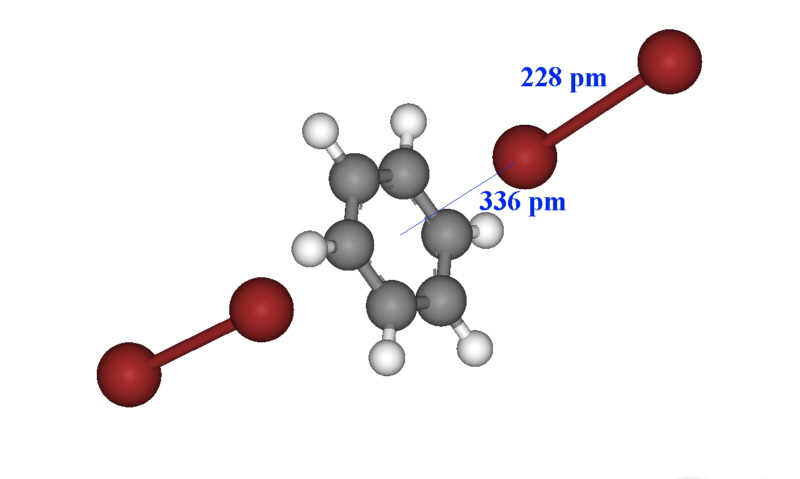
The X-ray structure of some of these have been obtained and often the intense colour can be used for characterisation and determination such as the bright blue colour of iodine in the presence of starch. In the case of the solid formed between dibromine and benzene, the structure is shown below and a new charge transfer band occurs at 292 nm. The Br-Br bond length is essentially unchanged from that of dibromine (228 pm).
In a study of the reaction of dibromine with substituted phosphines in diethyl ether, all but one showed a tetrahedral arrangement where one bromine was linked to the phosphorus.[3]
R3P + Br2 (Et2O, N2/r.t.) → R3PBr2The X-ray study of the triethylphosphine was interpreted as [Et3PBr]Br where the Br-Br separation was 330 pm. This is considerably longer than the 228 pm found above and was taken to mean that the compound was ionic In the case of the tri(perfluorophenyl)phosphine however the structure showed both bromines linked to give a trigonal bipyramid arrangement with D3 symmetry. Why (C6F5)3PBr2 was the only R3PBr2 compound that adopted trigonal bipyramidal geometry was reasoned to be due to the very low basicity of the parent tertiary phosphine.
Intermolecular forces are the attractive forces between molecules without which all substances would be gases. The various types of these interactions span large differences in energy and for the halogens and interhalogens are generally quite small. The dispersion forces involved in these cases are called London forces (after Fritz Wolfgang London, 1900-1954). They are derived from momentary oscillations of electron charge in atoms and hence are present between all particles (atoms, ions and molecules).
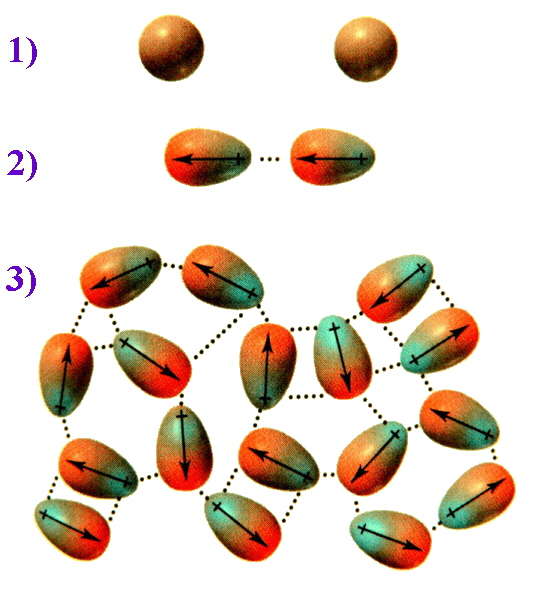
The ease with which the electron cloud of an atom can be distorted to become asymmetric is termed the molecule's polarizability. The greater the number of electrons an atom has, the farther the outer electrons will be from the nucleus, and the greater the chance for them to shift positions within the molecule. This means that larger nonpolar molecules tend to have stronger London dispersion forces. This is evident when considering the diatomic elements in group 17, the Halogens. All of these diatomic elements are nonpolar, covalently bonded molecules. Descending the group, fluorine and chlorine are gases, bromine is a liquid, and iodine is a solid. For nonpolar molecules, the farther you go down the group, the stronger the London dispersion forces.To picture how this occurs, compare the situation 1) where the electrons are evenly distributed and then consider 2) an instantaneous dipole that would arise from an uneven distribution of electrons on one side of the nucleus. When two molecules are close together, the instantaneous dipole of one molecule can induce a dipole in the second molecule. This results in synchronised motion of the electrons and an attraction between them. 3) Multiply this effect over numerous molecules and the overall result is that the attraction keeps these molecules together, and for diiodine is sufficient to make this a solid.
The changes seen in the variation of MP and BP for the dihalogens and binary interhalogens van be attributed to the increase in the London dispersion forces of attraction between the molecules. In general they increase with increasing atomic number.
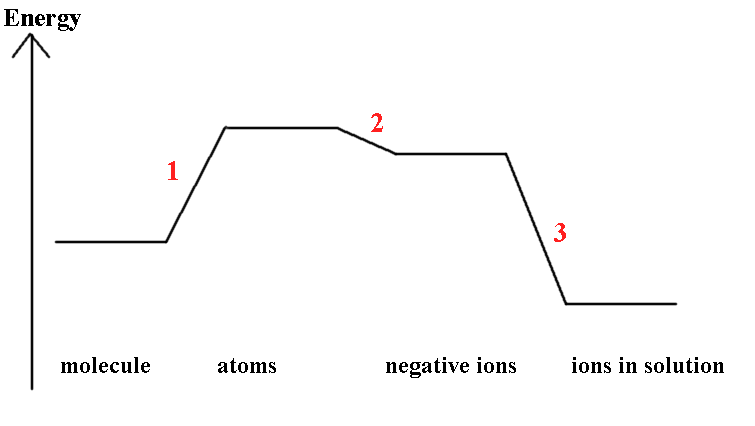
The redox potential, E°, X2/2X-, measures a free-energy change, usually dominated by the ΔH term. The values in the Table show that there is a decrease in oxidising strength proceeding down the group (2.87, 1.36, 1.09, 0.54 V). This can be explained by comparing the steps shown above.
1) Atomisation of the dihalide is the energy required to break
the molecule into atoms
½ X2(g) → X(g)
note that only F2 and Cl2 are gases in
their natural state so the energies associated with atomisation
of Br2 and I2 requires converting the
liquid or solid to gas first.
2) ΔEAH1 is the energy liberated when
the atom is converted into a negative ion and is related to the
Electron Affinity
X(g) + e- → X-(g)
Addition of an electron to the small F atom is accompanied by
larger e-/e- repulsion than is found for
the larger Cl, Br or I atoms. This would suggest that the process
for F should be less exothermic than for Cl and not fit the trend
that shows a general decrease going down the group.
3) ΔhydH°(X-,g) is the energy
liberated on the hydration of the ion, the Hydration
energy.
X-(g) + H2O →
X-(aq)
| Halogen | atomisation energy (kJ mol-1) |
ΔEAH1 (kJ mol-1) |
hydration enthalpy (kJ mol-1) |
overall (kJ mol-1) |
|---|---|---|---|---|
| F | +79.08 | -333 | -504 | -758 |
| Cl | +121.8 | -348 | -361 | -587 |
| Br | +111.7 | -324 | -330 | -542 |
| I | +106.7 | -295 | -285 | -473 |
This shows a very negative energy change for the fluoride compared to the others in the group. This comes about because of two main factors: the high hydration energy and the low atomisation energy. For F2 2) is less than for Cl2 but since the energy needed to break the F-F bond is also less and the hydration more, the total energy drop is much greater. In spite of their lower atomisation energies, Br2 and I2 are weaker oxidising agents than Cl2 and this is due to their smaller ΔEAH1 and smaller ΔhydH°.
It can be seen that the ΔEAH1 value for fluorine is in between those for chlorine and bromine and so this value alone does not provide a good explanation for the observed variation.
Each of the halogens is able to oxidise any of the heavier halogens situated below it in the group. They can oxidise hydrogen and nonmetals such as:
X2 + H2(g) → 2HX(g)In water, the halogens disproportionate according to:
X2 + H2O(l) → HX(aq) + HXO(aq), (where X=Cl, Br, I)3Cl2(g) + 6OH-(aq) → ClO3-(aq) + 5Cl-(aq) + 3H2O(l)
The trend seen for the complete removal of an electron from the gaseous halogen atoms is that fluorine has the highest IE1 and iodine the lowest. To overcome the attractive force of the nucleus means that energy is required so that the Ionisation Energies are all positive. The variation with size can be explained since as the size increases it take less energy to remove an electron. This inverse relationship is seen for all the groups, not just group 17. As the distance from the nucleus to the outermost electrons increases, the attraction decreases so that those electrons are easier to remove. The high value of IE1 for Fluorine is such that it does not exhibit any positive oxidation states, whereas Cl, Br and I can exist as high as 7.
Oxidation statesFluorine is the most electronegative element in the periodic table and exists in all its compounds in either the -1 or 0 oxidation state. Chlorine, bromine, and iodine however can be found in a range of oxidation states including: +1, +3, +5, and +7, as shown below.
| Oxidation States | Examples |
| -1 | CaF2, HCl, NaBr, AgI |
| 0 | F2, Cl2, Br2, I2 |
| 1 | HClO, ClF |
| 3 | HClO2, ClF3 |
| 5 | HClO3, BrF5, [BrF6]-, IF5 |
| 7 | HClO4, BrF6+, IF7, [IF8]- |
In general, odd numbered groups (like group 17) form odd-numbered oxidation states and this can be explained since all stable molecules contain paired electrons. (Free radicals are obviously much more reactive). When covalent bonds are formed or broken two electrons are involved so the oxidation state changes by 2.
When difluorine reacts with diiodine initially iodine monofluoride is formed. I2 + F2 → 2IF Return to Chemistry, UWI-Mona,
Home Page
Created and maintained by Prof. Robert J.
Lancashire,
Return to Chemistry, UWI-Mona,
Home Page
Created and maintained by Prof. Robert J.
Lancashire,