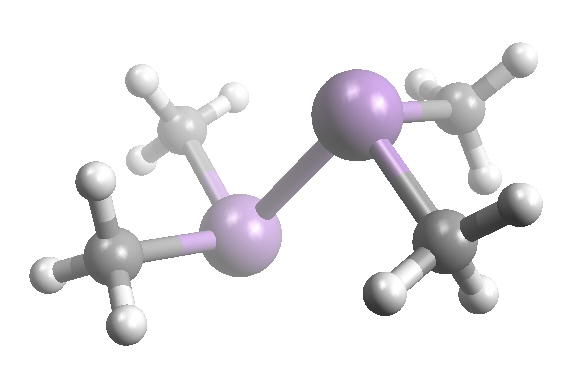
cacodyl
The French pharmacist-chemist, Louis-Claude Cadet de Gassicourt prepared what became known as "Cadet's fuming liquid" in 1757. The reaction involved heating 2 ounces of arsenious oxide with 2 ounces of potassium acetate.
As2O3 + 4 CH3COOK → As2(CH3)4O + 4 K2CO3 + CO2 → → As2(CH3)4
"a slightly coloured liquid of an extremely penetrating garlic odour distills and then a red-brown liquid which fills the receiver with thick fumes".
All of the early studies of Cadet's fuming liquid were qualitative in nature, made difficult by the liquid's horrible stench and inflammability, and it was not until the investigations of Robert Wilhelm Bunsen during 1837-1843 that more useful information concerning Cadet's fuming liquid became available.
The history of studies on this mixture was reviewed in 2001 [Ref 3.]Bunsen opted for a large-scale preparation, despite the fact that he was aware of the repulsive and dangerous nature of the expected products. Starting out with one kilogram of a 1:1 by weight As2O3/KOOCCH3 mixture in a glass retort, he heated it very slowly to red heat in a sand bath. As Cadet had reported, two liquid layers and a solid phase collected in the receiver. Bunsen reported that he obtained ~150 g of the red-brown liquid.
In 1841, Berzelius suggested to Bunsen that the name for the liquid be called "kakodyl" from the Greek meaning "stinking", the English spelling of this was cacodyl.
"Accidents with cacodyl compounds could have serious consequences. During his study of cacodyl cyanide, (CH3)2AsCN, prepared by reaction of 'cacodyl oxide' with a concentrated aqueous solution of mercuric cyanide, an explosion cost Bunsen the partial sight of his right eye and, as Roscoe reports, 'Bunsen was nearly poisoned, lying for days between life and death.' Bunsen recovered and completed his study of cacodyl cyanide, a most unpleasant compound. After distillation of the 'cacodyl oxide'/Hg(CN)2 reaction mixture, the cacodyl cyanide formed beautiful, prismatic crystals underneath the water layer. These were quite volatile (mp 32.5 °C). They were dried by pressing them between sheets of blotting paper. Bunsen noted that it is absolutely necessary to carry out this operation in the open air while breathing through a long glass tube that extends to fresh air far beyond the volatile crystals. And well might this compound be avoided! Bunsen reported that the vapour from 1 grain (0.0648 g) of cacodyl cyanide in a room produces sudden numbness of the hands and feet, and dizziness and insensibility to the point of unconsciousness. The tongue becomes covered with a black coating. These effects, however, are only temporary, with no lasting problems. (Bunsen, it may be noted, lived to the ripe old age of 88.)" Ref 3.
Seventy years after its discovery, the question of the composition of Cadet's fuming liquid was addressed by Valeur and Gailliot by means of its fractional distillation under an atmosphere of CO2. Valeur, A.; Gailliot, P. C. R. Acad. Sci. 1927, 185, 956.
| Compound | % in Cadet's liquid | melting point | boiling point | density |
| (CH3)3As | 2.6 | liquid at -80°C | 50°C | 1.144 |
|
(CH3)2AsOAs(CH3)2 "cacodyl oxide" |
40 | -57°C | 150°C | 1.486 |
|
(CH3)2As-As(CH3)2 "cacodyl" |
55.9 | -5°C | 163°C | 1.447 |
|
Me7As3 and Me5As3 (mixture) |
1.3 | very viscous at -80°C | 115-120°C /5 mmHg |
1.647 |
| (CH3As)5 | 0.2 | 10°C | 190°C /5 mmHg |
2.15 |
The use of Cadet's fuming liquid was considered for use in chemical warfare during both WWI and WWII and plants in both Germany and the USA were said to have developed processes for large-scale production. During WWI an organoarsenic compound was used ( Lewisite) but not Cadet's liquid.
The exact composition of Cadet's fuming liquid is still unclear but with todays array of sophisticated spectroscopic instruments it should be possible to find a definitive answer. The problems of toxicity etc. are no longer an insurmountable problem given the handling techniques and glassware that were developed beginning with the experimental work of the early synthetic chemists like Bunsen and Schlenk.
"Gosio gas"Another "simple" methyl derivative, Me3As, has a long history as well. In 1893 the Italian physician Bartolomeo Gosio published his results on "Gosio gas" that was subsequently shown to contain trimethylarsine. Under wet conditions, the mould Scopulariopsis brevicaulis produces significant amounts of methyl arsines via methylation of arsenic-containing inorganic pigments, especially Paris green/Schweinfurt-green("copper arsenite plus copper acetate") and Scheele's Green ("copper arsenite") which were once used in indoor wallpapers. In other cases the arsenic had been added to the wallpaper paste to discourage rodents and insects. Gosio gas was responsible for a number of deaths, and the air in the buildings in which it was being produced had a characteristic garlic-like odour. Gosio established the source of the problem and isolated some of the moulds capable of metabolizing inorganic arsenicals. Pietro Biginelli aspirated the gas from cultures through acidified (HCl) mercuric chloride solution. On the basis of an analysis of the precipitate so obtained, he incorrectly identified the gas as diethylarsine (Et2AsH). Nonetheless, this was a considerable achievement and established the methodology that Frederick Challenger was to use some 30 years later in his classic studies, beginning with the positive identification of the mould metabolite as trimethylarsine.
Newer studies suggest that trimethylarsine has a lower toxicity than originally thought and may not account for the death and the severe health problems observed in the 19th century which may have arisen due to volatile organics produced from moulds (now linked to what has been called "sick-building syndrome")
Lead arsenate dust from a painted ceiling was the source of the arsenic that caused health problems for Clare Boothe Luce when she was living in Rome as U.S. Ambassador in 1954. No mould action was implicated. One theory was that a washing machine in an upstairs room caused vibrations that dislodged some arsenic containing paint from the stucco decorating her bedroom ceiling!
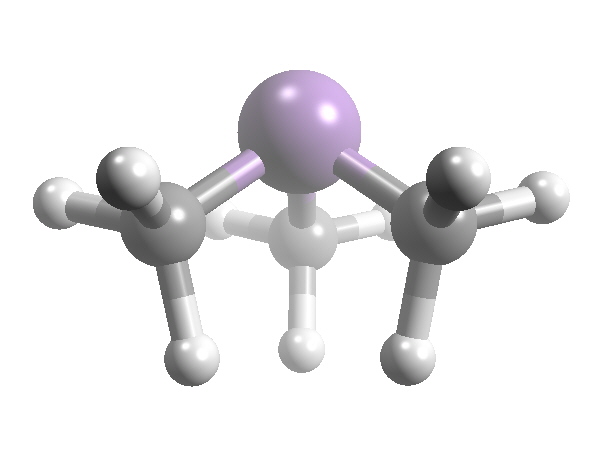
AsMe3 is a pyramidal molecule as predicted by VSEPR theory. The As-C distances average 151.9 nm, and the C-As-C angles are 91.83°
References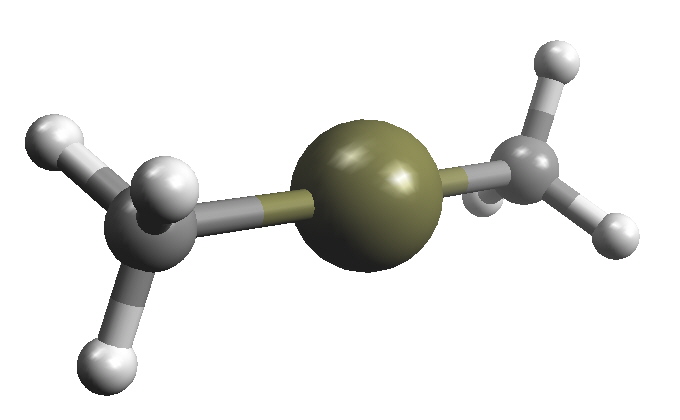
Mercury is 62nd in terms of natural abundance and is found everywhere, usually as the mineral cinnabar, HgS, although 30 minerals containing Hg are known. The oxidation states are Hg(0), Hg(I) and Hg(II), where Hg(I) has been shown to exist as Hg22+.
The three Hg species are related by the disproportionation:There have been several serious outbreaks of mercury
poisoning. The most famous was between 1953 and 1965 at Minamata
Bay in Japan when 46 people died and 120 suffered severe
symptoms. As of March 2001, 2,265 victims had been officially
recognised (1,784 of whom had died) and over 10,000 had received
financial compensation from Chisso. By 2004, Chisso Corporation
had paid $86 million in compensation, and in the same year was
ordered to clean up its contamination. On March 29th, 2010, a
settlement was reached to compensate as-yet uncertified
victims.
The disease was first noticed in cats (who were seen throwing
themselves into the sea) and was quickly traced to mercury
poisoning acquired as a result of eating contaminated fish (5-10
ppm Hg). The investigations that followed showed that the fish
had acquired the high mercury due to the dumping of inorganic
mercury salts and methylmercury from the Chisso Co. plastics
factory upstream.
Analysis of fish exhibits from museums, some over 90 years old, has shown that mercury levels for ocean fish are similar but that river fish levels have risen as a result of man-made contamination. The forms of mercury occuring in the environment are Hg2+ and methylmercury, either MeHg+ or Me2Hg. Interconversion can be affected by microorganisms.
Aerobes can solubilise Hg2+ from cinnabar
(Ksp ~10-53) which in sediments was
considered safe since the solubility product was so small. The
conversion of S2- → SO32-
→ SO42- allows the insoluble sulfide
to breakdown and in the process other Hg(II) salts are formed or
the mercury may get reduced to Hg(0) enzymatically.
Hg2+ + NADH + H+ → Hg0 +
NAD+ + 2 H+, where NADH = reduced form of
nicotinamideadeninedinucleotide
This conversion can be considered as a detoxification process
since Hg0 is more easily eliminated.
In the environment, sulfate-reducing bacteria take up mercury in its inorganic form and through metabolic processes convert it to methylmercury. Sulfate-reducing bacteria are found in anaerobic conditions, typical of the well-buried muddy sediments of rivers, lakes, and oceans where methylmercury concentrations tend to be highest. Sulfate-reducing bacteria use sulfur rather than oxygen as their cellular energy-driving system. One hypothesis is that the uptake of inorganic mercury by sulfate-reducing bacteria occurs via passive diffusion of the dissolved complex HgS. Once the bacterium has taken up this complex, it utilizes detoxification enzymes to strip the sulfur group from the complex and replaces it with a methyl group:
HgS → CH3Hg(II)X + H2SThe major source of methylmercury exposure in humans is consumption of fish, marine mammals, and crustaceans. Once inside the human body, roughly 95% of the fish-derived methylmercury is absorbed from the gastrointestinal tract and distributed throughout the body. Uptake and accumulation of methylmercury is rapid due to the formation of methylmercury-cysteine complexes. Methylmercury is believed to cause toxicity by binding the sulfhydryl groups at the active centers of critical enzymes and structural proteins. Binding of methylmercury to these moieties constitutively alters the structure of the protein, inactivating or significantly lowering its functional capabilities.
Once the Me2Hg is formed it is volatile and when released into the atmosphere it is readily photolysed by UV lightOrganic mercury tends to increase up the food chain, particularly in lakes. The mud at the bottom of a lake may have 100 or 1000 times the amount of mercury than is in the water. Bacteria, worms and insects in the mud extract and concentrate the organic mercury. Small fish that eat them further concentrate the mercury in their bodies. This concentration process, known as "bioaccumulation", continues as larger fish eat smaller fish until the top predator fish in the lake may have methylmercury levels in their tissues that are up to 1,000,000 times the level in the water in which they live. We then eat the fish....

To consume a human being would be extremely unhealthy for any animal. Humans carry the highest concentration of toxic chemicals of all creatures on the planet. Their livers, hearts, kidneys and brains are so heavily contaminated with hundreds of different synthetic chemicals that if humans were slaughtered as a meat source, they'd never pass USDA food safety standards.from Hg in Humans
| Molecular formula | C2H6Hg |
| Molar mass | 230.66 g mol-1 |
| Appearance | Colourless liquid |
| Density | 2.96 g/mL |
| Melting point | -43 °C |
| Boiling point | 87-97 °C |
| Solubility in water | Insoluble |
Like mercury, lead is primarily obtained from its sulfide ore, in this case Galena, PbS, yet once again there are quite a number of other minerals containing lead. In terms of natural abundance it exists at about 14 ppm in the Earth's crust (37th compared to O), however it has become well known due to its ease of extraction and the number of uses with technical importance.
"the drinking of lead causes oppression to the stomach, belly and intestines with wringing pains; it suppresses the urine, while the body swells and acquires and unsightly leaden hue".
The possible hazards associated with the use of lead piping in water systems was recognised as long ago as the first century BC and it has even been suggested that the "decline of the Roman Empire" might have been ascribed to the use of lead acetate as an additive to sweeten wine. It is somewhat surprising therefore that the first legislation controlling the industrial hazards of lead industries was not introduced until 1864.
Note that Dr. Wilton Turner (born in Clarendon, Jamaica in the early 1800's) wrote on the inappropriate use of lead in sugar and rum production while running a rum distillery in Guyana. One advocate said he had fed lead to dogs and guinea pigs for several weeks and seen no adverse affects in fact the guinea pigs were stolen which he thought was because they looked so fat and healthy!
An examination (in the early 1970's) of the annual snow strata in Northern Greenland and Poland revealled most elegantly that levels in air-borne lead had increased significantly since the Industrial Revolution and very sharply since 1940. Considering that 40-50% can be absorbed by inhalation compared to only 5-10% through ingestion this was cause for concern.
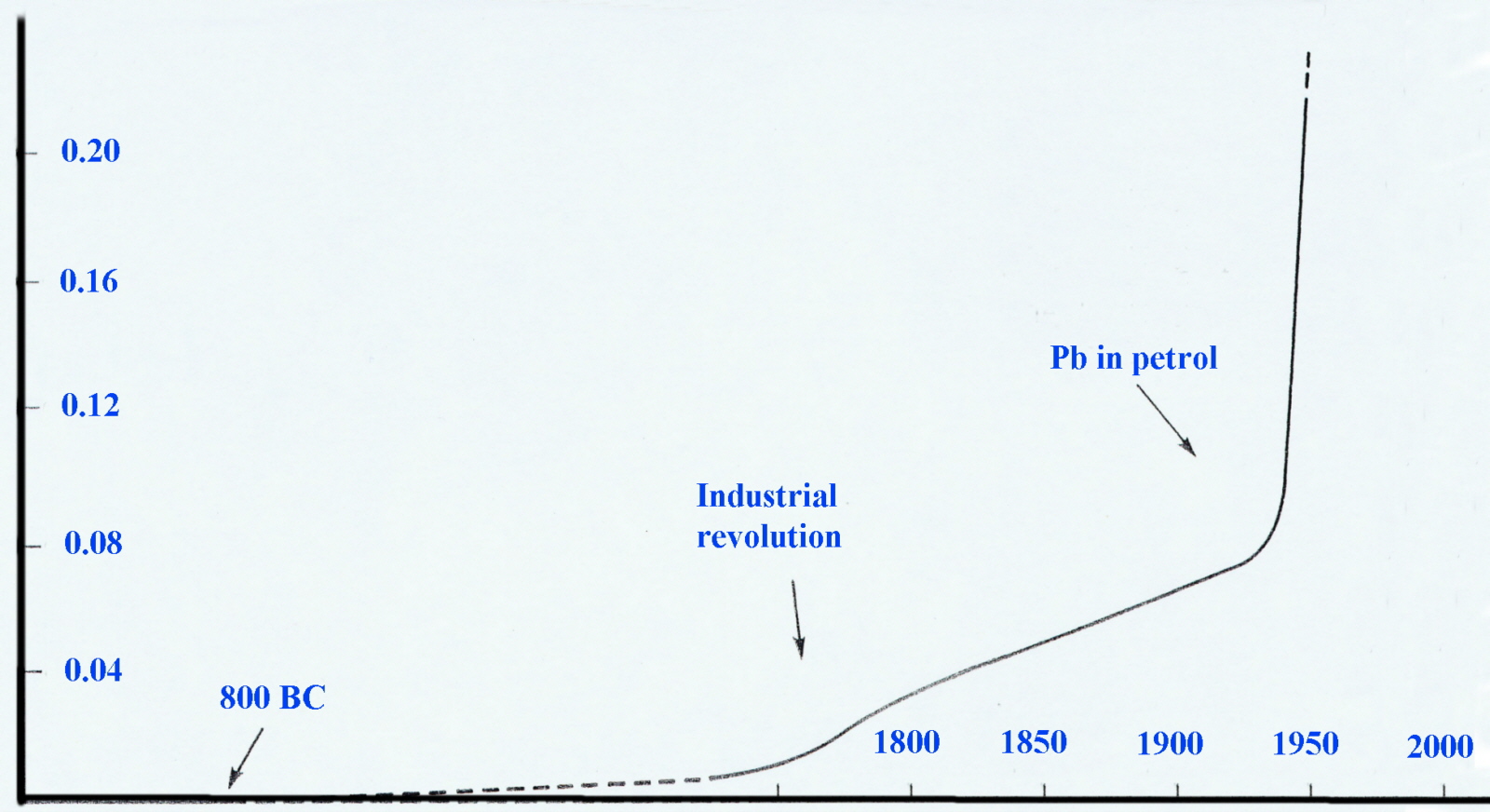
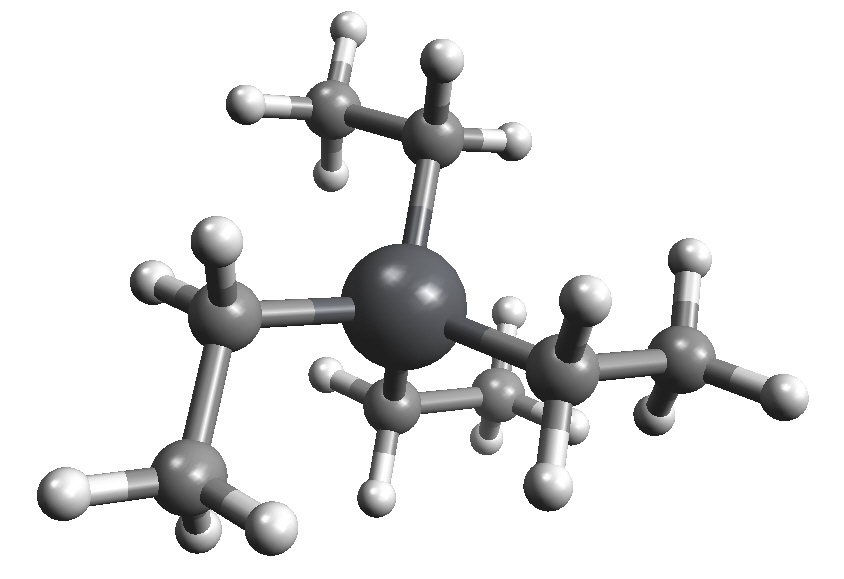
Leaded gasoline was an economic success from 1926 until 1976, and in fact, its discovery by Thomas Midgley at Charles Kettering's General Motors laboratory was among the most celebrated achievements of automotive engineering. It was often portrayed as the result of genius, luck and a great deal of hard work. It is now considered to be a catastrophic failure and is banned for environmental and public health reasons. {There are still a few countries selling petrol with lead additives.} Even more surprising is that the use of ethanol in fuel was already well established by the time tetraethyllead was introduced as an additive.
Tetraethyllead was supplied for mixing with raw gasoline in the form of "ethyl fluid", which was Et4Pb blended together with the lead scavengers 1,2-dibromoethane and 1,2-dichloroethane. "Ethyl fluid" also contained a reddish dye to distinguish treated from untreated gasoline and discourage the use of leaded gasoline for other purposes such as cleaning.
Ethyl fluid was added to gasoline in the ratio of 1:1260, usually at the refinery. The purpose was to increase the fuel's octane rating. A high enough octane rating is required to prevent premature detonations known as engine knocking ("knock" or "ping"). Antiknock agents allow the use of higher compression ratios for greater efficiency and peak power. The formulation of "ethyl fluid" was:Humans have been mining and using this heavy metal for thousands of years, poisoning themselves in the process. Although lead poisoning is one of the oldest known work and environmental hazards, the modern understanding of the small amount of lead necessary to cause harm did not come about until the latter half of the 20th century. No safe threshold for lead exposure has been discovered, that is, there is no known amount of lead that is too small to cause the body harm.
Lead pollution from engine exhaust is dispersed into the air and into the vicinity of roads and easily inhaled. Lead is a toxic metal that accumulates and has subtle and insidious neurotoxic effects especially at low exposure levels, such as low IQ and antisocial behavior. It has particularly harmful effects on children. These concerns eventually led to the ban on Et4PB in automobile gasoline in many countries. For the entire U.S. population, during and after the Et4PB phaseout, the mean blood lead level dropped from 13 µg/dL in 1976 to only 3 µg/dL in 1991. The U.S. Centers for Disease Control considered blood lead levels "elevated" when they were above 10 µg/dL. Lead exposure affects the intelligence quotient (IQ) such that a blood lead level of 30 µg/dL is associated with a 6.9-point reduction of IQ, with most reduction (3.9 points) occurring below 10 µg/dL.
Also in the U.S., a statistically significant correlation has been found between the use of Et4PB and violent crime: taking into account a 22-year time lag, the violent crime curve virtually tracks the lead exposure curve. After the ban on Et4PB, blood lead levels in U.S. children dramatically decreased.
Even though leaded gasoline is largely gone in North America, it has left high concentrations of lead in the soil adjacent to all roads that were constructed prior to its phaseout. Children are particularly at risk if they consume this, as in cases of pica.
Note as well the work done in 1995 by ICENS on the problem of the old disused lead mine and tailings that affected school children in Kintyre. Over 40 cases were detected with unacceptable levels. ICENS at that time cleaned the community and sought to educate residents about the dangers. A continuation of the research done in Kintyre was to test 628 children at 17 basic schools across the island. Children at a number of basic schools in Kingston and St Catherine were discovered with blood lead levels as low as 45 µg/dL and as high as 60. In two of the cases, children had lead levels of 130 and 202. At this level, they would likely die from the poisoning if untreated.
Properties of Et4Pb| Molecular formula | C8H20Pb |
| Molar mass | 323.44 g mol-1 |
| Appearance | Colourless, viscous liquid |
| Density | 1.653 g/mL (20 °C) |
| Melting point | -136 °C |
| Boiling point | 84-85 °C/15 mm Hg |
| Solubility in water | Insoluble |
 Return to Chemistry, UWI-Mona,
Home Page
Created and maintained by Prof. Robert J.
Lancashire,
Return to Chemistry, UWI-Mona,
Home Page
Created and maintained by Prof. Robert J.
Lancashire,