Stability
When discussing the concept of Stability it is necessary to
distinguish between thermodynamic and kinetic stability.
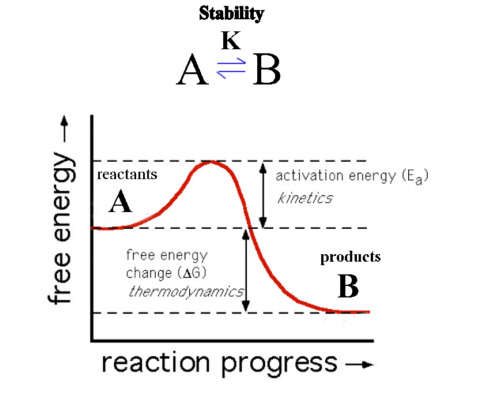
Here B is at lower energy than A so that ΔG is negative.
The reaction should therefore proceed spontaneously and B is the
more thermodynamically stable species.
The reaction as shown though has a barrier to the progress of the
reaction called the Activation Barrier (Ea) and so the reaction
may proceed very slowly. The thermodynamics describes only the
starting and ending position of the reaction and not the
intermediate or transition state. If the kinetics is slow, A is
described as being inert while if it proceeds quickly then A is
described as being labile.
The conditions that distinguish them are:
if the reaction takes longer than 1 minute under the conditions of
concentration 0.1 M, temperature 25°C, then it is INERT,
if under the same conditions the reaction time < 1 minute, then it is LABILE.
In the lecture on isomerism,
we depend on the samples being kinetically stable i.e. inert.
In the lecture on Chelation and Stability
we concentrate on thermodynamic stability and look at the changes in free energy,
enthalpy and entropy during the reaction.
When we consider thermodynamic stability we need to be familiar
with 2 formulae:
ΔG⦵ = - RT ln(K) or alternatively
ΔG⦵ = - 2.303RT log10(K) ---(1)
ΔG⦵ = ΔH⦵ - TΔS⦵ ---(2)
The first relates the free energy to the stability constant and the second
shows the breakdown into the component enthalpy and entropy terms.
Return to Coordination Chemistry Course
Outline.
 Return to Chemistry, UWI-Mona,
Home Page
Return to Chemistry, UWI-Mona,
Home Page
Copyright © 2006-2015 by Robert John
Lancashire, all rights reserved.
Created and maintained by Prof. Robert J.
Lancashire,
The Department of Chemistry, University of the West Indies,
Mona Campus, Kingston 7, Jamaica.
Created 2006. Links checked and/or last modified
30th March 2015.
URL
http://wwwchem.uwimona.edu.jm/courses/IC10Kstability.html

 Return to Chemistry, UWI-Mona,
Home Page
Return to Chemistry, UWI-Mona,
Home Page