The Irving-Williams
series
The general stability sequence of high spin octahedral metal complexes for
the replacement of water by other ligands is:
Mn(II) < Fe(II) < Co(II) < Ni(II) < Cu(II) >
Zn(II)
This trend is essentially independent of the ligand.
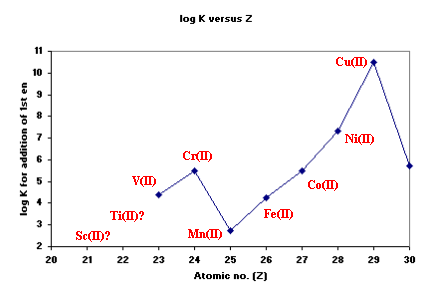
In the case of 1,2-diaminoethane (en), the first step-wise
stability constants (logK1) for M(II) ions are shown below.
Notes
The sequence is generally quoted ONLY for Mn(II) to Zn(II) since there is
little data available for the other first row transition metal
ions; their M(II) oxidation states are not very stable.
The position of Cu(II) is considered out-of-line with predictions
based on Crystal Field Theory and is probably a consequence of the
fact that Cu(II) often forms distorted octahedral complexes.
(See notes on the Jahn-Teller effect).
One explanation
Crystal Field Theory is based on the idea that a purely electrostatic
interaction exists between the central metal ion and the ligands.
This suggests that the stability of the complexes should be related
to the ionic potential; that is, the charge to radius ratio.
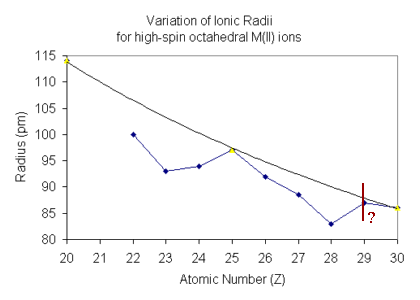
In the Irving-Williams series, the trend is based on high-spin M(II) ions,
so what needs to be considered is how the ionic radii vary across the
d-block.
For free metal ions in the gaseous phase it might be expected that
the ionic radius of each ion on progressing across the d-block
should show a gradual decrease in size. This would come about due
to the incomplete screening of the additional positive charge by the
additional electron, as is observed in the Lanthanide Contraction.
For high-spin octahedral complexes it is essential to consider
the effect of the removal of the degeneracy of the d-orbitals
by the crystal field. Here the d-electrons will initially add
to the lower t2g orbitals before filling the eg orbitals
since for octahedral complexes, the t2g subset are directed
in between the incoming ligands whilst the eg subset are directed
towards the incoming ligands and cause maximum repulsion.
For d1-d3 (and d6-d8)
the addition of the electrons to the
t2g orbitals will mean that the screening of the increasing
attractive nuclear charge is not very effective and the radius
should be smaller than for the free ion.
The position of d4 and d9 on the plot is difficult
to ascertain with certainty since six-coordinate complexes are expected
to be distorted due to the Jahn-Teller Theorem.
Cr(II) is not very stable so few measurements are available. For Cu(II)
however, most complexes are found to have 4 short bonds and 2 long bonds
although 2 short and 4 long bonds is feasible.
The radii are expected to show an increase over the
d3 and d8 situation since electrons are being
added to the eg subset. The reported values have been found to
lie on both sides of the predicted value.
For d0, d5 and d10 the screening expected
is essentially that of a spherical arrangement equivalent to the
absence of a crystal field. The plot above shows that these points
return to the line drawn showing a gradual decrease of the radius on
moving across the d-block.
Once the decrease in radius with Z pattern is understood, it is a
small step to move to a pattern for q/r since this only involves taking the
reciprocal of the radius and holding the charge constant. The radius essentially
decreases with increasing Z, therefore 1/r must increase with
increasing Z.
For the sequence Mn(II) to Zn(II), the crystal field (q/r) trend expected
would be:
Mn(II) < Fe(II) < Co(II) < Ni(II) > Cu(II) >
Zn(II)
Apart from the position of Cu(II), this corresponds to the Irving-Williams
series. The discrepancy is once again accounted for by the fact that
copper(II) complexes are often distorted or not octahedral at all.
When this is taken into consideration, it is seen that the Irving-Williams
series can be explained quite well using Crystal Field Theory.
return to the C21J course
outline
 Return to Chemistry, UWI-Mona,
Home Page
Return to Chemistry, UWI-Mona,
Home Page
Copyright © 2000-2010 by Robert John
Lancashire, all rights reserved.
Created and maintained by Prof. Robert J.
Lancashire,
The Department of Chemistry, University of the West Indies,
Mona Campus, Kingston 7, Jamaica.
Created November 2000. Links checked and/or
last modified 1st September 2010.
URL
http://wwwchem.uwimona.edu.jm/courses/IrvWill.html

 Return to Chemistry, UWI-Mona,
Home Page
Return to Chemistry, UWI-Mona,
Home Page