|
Spinel is the name given to the mineral
MgAl2O4. It has a common structural
arrangement shared by many oxides of the transition metals
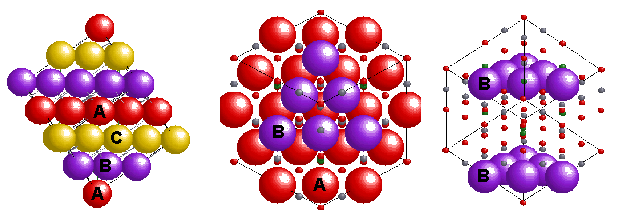
with formula AB2O4. In the normal pattern the oxygens (red) form a cubic close packed (ABCABC or face centred) array and the Mg(II) (A(II)-green) and Al(III) (B(III)-silver) sit in tetrahedral (1/8 occupied) and octahedral (1/2 occupied) sites in the lattice, giving a Unit Cell with 8 Mg's, 16 Al's and 32 O's. An inverse spinel is an alternative arrangement where the divalent ions swap with half of the trivalent ions so that the M(II) now occupy octahedral sites i.e. B(AB)O4. In this case Ni(II) is octahedral and half of the Fe(III) are tetrahedral. Complexes that share this structure include.. a number of 1st row TM oxides and sulfides. |

 Return to Chemistry, UWI-Mona,
Home Page
Return to Chemistry, UWI-Mona,
Home Page
Copyright © 2000-2010 by Robert John Lancashire, all rights reserved.
Created and maintained by Prof. Robert J. Lancashire,