Experiment 2: Calibration of a pipette
Introduction
An ordinary laboratory pipette may be expected to deliver
its nominal volume with good precision and good accuracy if
it is used in the way recommended. In this experiment we
investigate the precision and accuracy of such a pipette by
making accurate determinations of the mass of water it delivers
in repeated operations.
Materials
10.0-cm3 pipette, 50-cm3 beaker,
250-cm3 Erlenmeyer flask, thermometer, pipette filler, graph paper.
Procedure (Read carefully)
- Clean the beaker and the pipette and dry the beaker.
- Obtained distilled water in the Erlenmeyer flask and let it
stand on the bench for about 15 min before determining its
temperature.
- Weigh the beaker on a balance which allows you to determine
the mass to the nearest tenth of a milligram (i.e. ± 0.001
g).
- Fill (use the pipette filler) and discharge the pipette as
recommended, into the beaker, and determine the mass of water
discharged by taking the difference.
- Repeat step 4 until you have the results of four (4) such
trials.
Note: You do not need to empty and dry the beaker
between trials. You should try to spend no more than a
half-hour in the balance room so that everyone will have
an opportunity to use the balances in the time allotted.
- Determine the temperature of the water you have pipetted and
take the mean of the two temperatures you have measured as the
effective temperature of the water during the calibration.
Calculations
1. Find the mean of the four trials. The mean volume can
now be determined from the mean mass and the density of water at
the temperature you determined in 6 above. However, there is a
correction to be made for the upthrust of air on the water during
the weighing. In normal day-to-day use of balances we neglect
this correction, but now that we are interested in accurate
calibration, we will take this into account. The correction
occurs because the upthrust of air on the water is greater than
the upthrust on the balance weights used in our balances.
This correction is in effect, 1.06 mg for every gram of
water weighed, (when it is assumed that the density of air is
0.0012 g cm3 and the density of stainless steel
weights is 8.4 g cm3).
2. Correct the mean mass for air buoyancy by adding to the
mean mass, 1.06 mg per gram of water discharged by the
pipette.
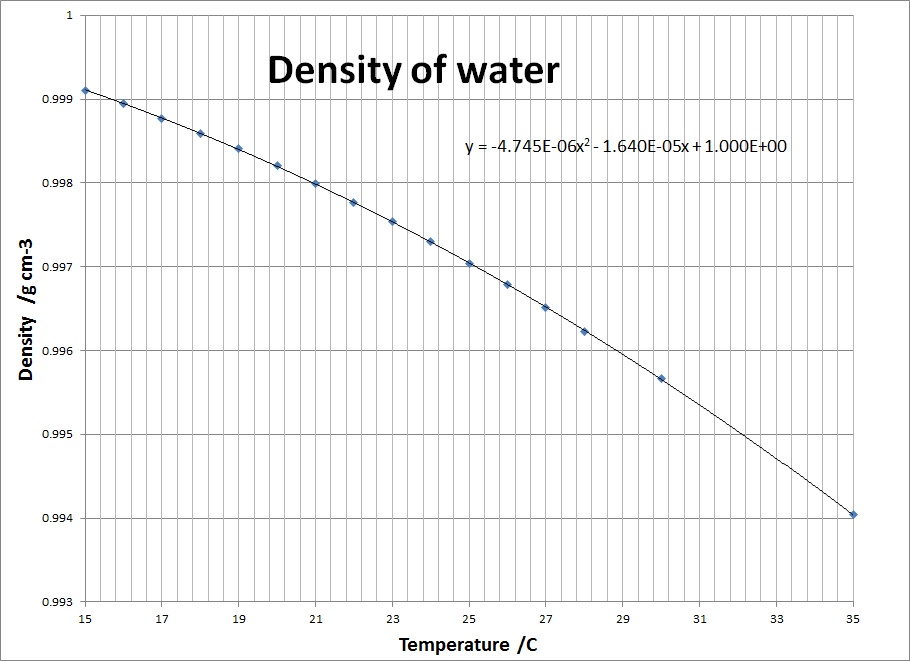
3. Determine the density of water, at the temperature
measured in 6 above, from the tables given.
4. Find the mean volume of water discharged by the pipette
using the formula:
Vo = mass / density
5. Comment on the accuracy of the pipette and show your
results to a demonstrator, who will initial the results and make
a note of your value.
6. We now want to determine the precision of the pipette,
but do not have enough data points to do rigorous statistical
analysis. The demonstrator will put you in touch with someone
with a pipette with volume fairly close to yours. Copy this
person's four data points and perform the statistical analysis to
find the standard deviation of the eight data points. You may
neglect the correction for air buoyancy in doing the statistical
analysis, but you should find a new mean volume for your eight
data points.
Table of Mass and Volume of Water obtained in 8 Pipette
Trials
| Trial No. |
Mass of water/g |
Vol. of water, Vi/cm3 |
(Vi - Vavg) = di |
(Vi - Vavg)2 = di2 |
| 1 |
|
|
|
|
| 2 |
|
|
|
|
| 3 |
|
|
|
|
| 4 |
|
|
|
|
| 5 |
|
|
|
|
| 6 |
|
|
|
|
| 7 |
|
|
|
|
| 8 |
|
|
|
|
The standard deviation, σ, is given by the formula:
σ= √ {∑(di)2 / (N-1)}
7. Write your result for the volume you determined in 4
with the error you have now estimated from the data;
Volume of pipette = Vo (± s)
Comment now, on the accuracy and precision of the pipette.
 Return to Chemistry, UWI-Mona,
Home Page
Return to Chemistry, UWI-Mona,
Home Page
Copyright © 2002-2014 by The Department
of Chemistry UWI, Jamaica, all rights reserved.
Created and maintained by Prof. Robert J.
Lancashire,
The Department of Chemistry, University of the West Indies,
Mona Campus, Kingston 7, Jamaica.
Created Oct 2002. Links checked and/or last
modified 14th September 2014.
URL
http://wwwchem.uwimona.edu.jm/lab_manuals/c10expt2.html

 Return to Chemistry, UWI-Mona,
Home Page
Return to Chemistry, UWI-Mona,
Home Page