Experiment 8 - Separation of an Unknown Mixture by Acid/Base Extraction
Experimental Aims: The objective of this
exercise is to separate a two-component mixture using extraction
techniques and then to identify the isolated components by
determining their melting points.
Experimental learning objectives: At the end of
this experiment you should be able to: (i) use a
separatory/dropping funnel; (ii) dry an organic liquid; (iii) use
a rotary evaporator; (iv) identify the organic phase in an
immiscible organic/aqueous mixture; (v) use acid/base reactions
to impact the solubility of organic compounds and (vi) determine
melting points.
Each student will be given a mixture of two substances, which
belong to two of the three categories listed below.
| Possible carboxylic acids |
benzoic acid |
2-chlorobenzoic acid |
| Possible phenols |
4-tert-butylphenol |
2-naphthol |
| Possible neutrals |
1,4-dimethoxybenzene |
fluorene |
Background Reading: Besides reading the lab
manual you will need to do a little bit more. To help you
understand the chemical basis of this exercise, you should review
Sections 3.5 - 3.7 in Solomons & Fryhl which concerns the
properties of acids and their conjugate bases. Pay particular
attention to the use of pKa values. You
should also review the appropriate pages in the Mohrig and pay
keen attention during your lab talk to acquaint yourself with
extraction, washing , drying agents, and recrystallization.
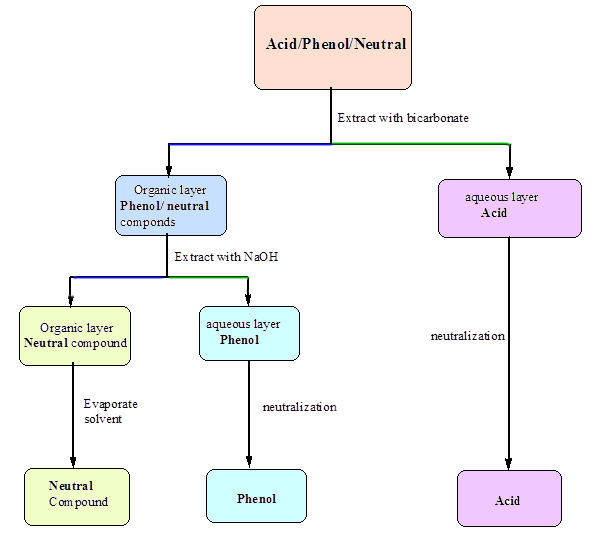
Background Information: Extraction is a
particularly useful means of separating organic compounds if one
compound in the mixture can be chemically converted to an ionic
form. The ionic form is soluble in an aqueous layer and can be
extracted into it. Other, non-ionic organic compounds in the
mixture will remain dissolved in the organic solvent layer.
Separation of the two layers results in the separation of the two
compounds.
The extent to which an acid-base reaction proceeds to
completion depends upon the relative acidity of the reactants and
products. Reactions occur so that stronger acids and
bases are converted into weaker conjugate base and conjugate
acids, respectively. The pKa value of the
acids gives a measure of the acidity of each compound. Stronger
acids have smaller pKa values and their conjugate bases
are weaker. The position of an acid-base equilibrium can then be
predicted from knowledge of the pKa values of the acids
involved.
Take a look at the following acid-base reactions in Figure 1, paying
particular attention to the position of the equilibrium and
its relationship to the pKa values given.
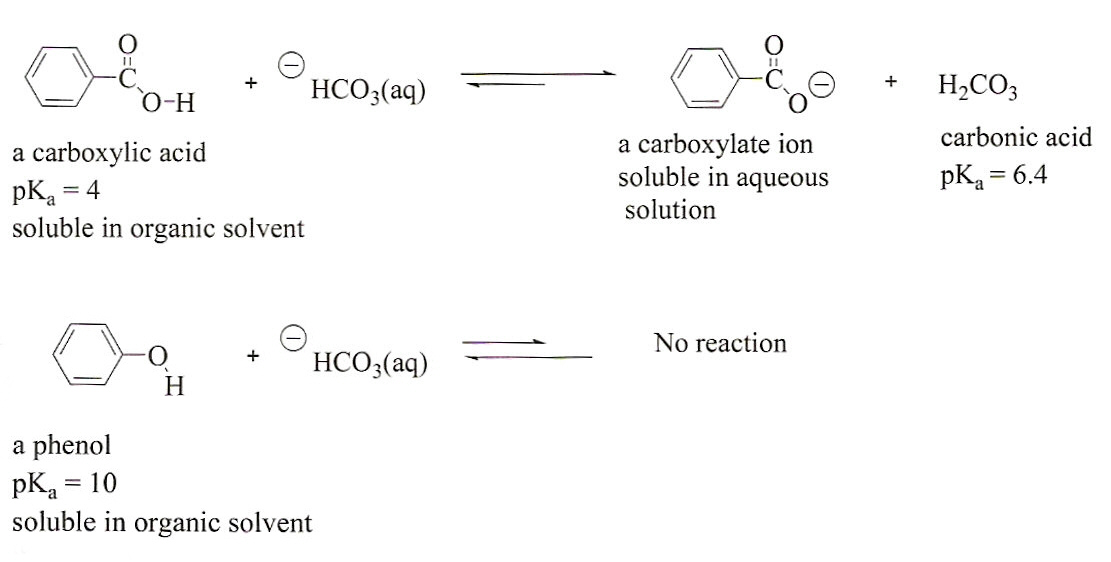 Figure 1:
Figure 1:
The reactions of a carboxylic acid and
a phenol with bicarbonate ion. Note that the carboxylic acid has
a lower pKa than the conjugate acid of bicarbonate ion (carbonic
acid). The reaction, therefore, proceeds to products. The
reaction of a phenol, however, favors the reactants since the pKa
of phenol (10) is larger than that of the carbonic acid (6.4).
Acid-base reactions favor the side with the weaker acid (that is,
they favor the side with the larger pKa). So, extracting a
mixture of these two compounds with bicarbonate results in the
ionization and extraction of a carboxylic acid in the presence of
phenol thus separating the two compounds from one another.
Now, look at the reaction in Figure 2 where we use a stronger
base to do the reaction:
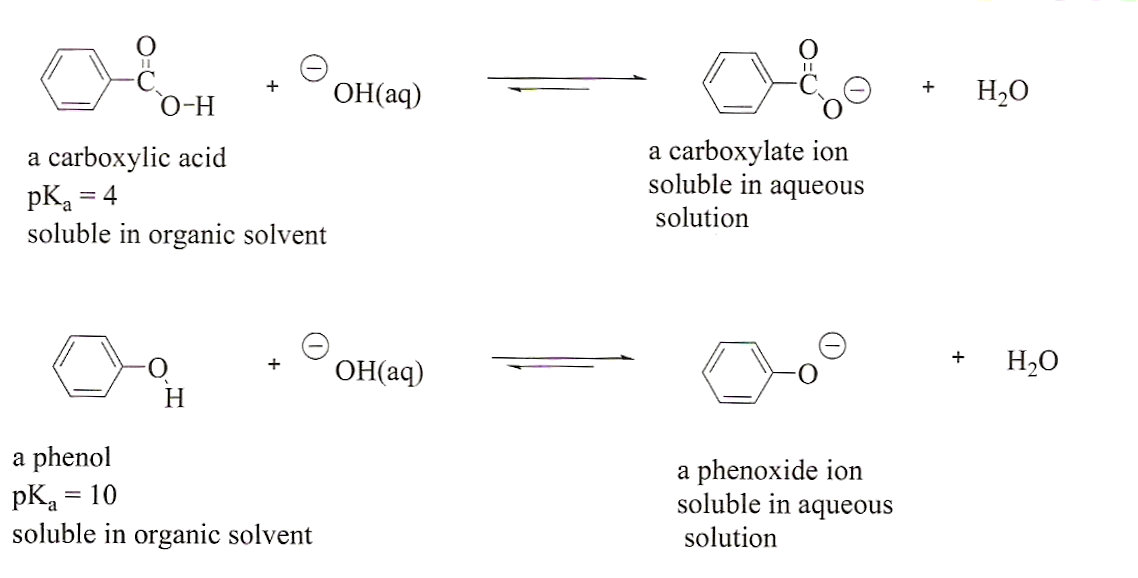 Figure 2:
Figure 2:
The reactions of a carboxylic acid and
a phenol with hydroxide ion. Note that in both cases, the
reactions favor the formation of products. Therefore, extracting
with hydroxide ion would result in the ionization and extraction
of both compounds at he same time.
A close look at these two figures indicates that separating a
mixture of a carboxylic acid and a phenol would best be done
using bicarbonate ion since only the carboxylic acid is converted
into its conjugate base by bicarbonate. The conjugate base of the
carboxylic acid, being an ionic species, is soluble in the
aqueous layer while the phenol (left unionized) would remain
dissolved in the organic layer. However, if we were to extract
with hydroxide ion, both the carboxylic acid and the phenol would
be converted into their conjugate bases (see figure2). The
conjugate bases, again are both ionic species and therefore
soluble in the aqueous layer. This means that both compounds
would be extracted at the same time, resulting in no separation.
A neutral compound will not react with either bicarbonate ion or
hydroxide ion since a neutral compound does not have protons
acidic enough to be removed by these bases. Therefore, such a
compound will remain dissolved in the organic layer, no matter
which base is added. For example, a mixture of neutral compound
and a carboxylic acid can be separated using bicarbonate ion
since only carboxylic acid will be ionized by the bicarbonate
ion.
Once extracted, the carboxylic acid and phenol can both be
recovered by adding HCl to the aqueous solutions. The carboxylate
ion and phenoxide will both be protonated by HCl, resulting in
the formation of the original carboxylic acid and phenol, neither
of which is soluble in water so they precipitate from solution.
The solid can then be isolated by filtration. Figure 3 shows this
chemistry for you.
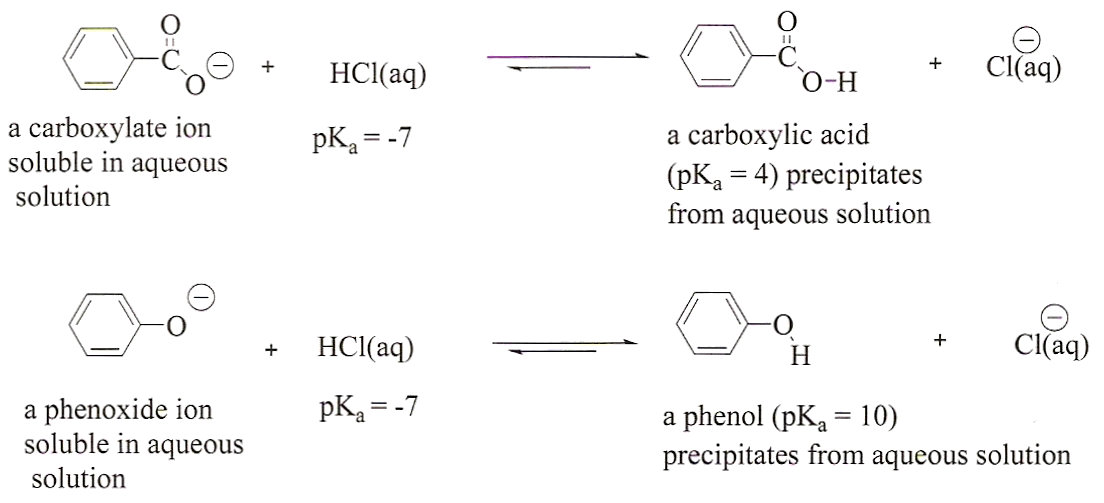 Figure 3:
Figure 3:
The reactions of a carboxylate ion and
a phenoxide ion with HCl. Since HCl is stronger acid than either
of the conjugate acids, the products are favored in both cases.
The products, a carboxylic acid and a phenol, are insoluble in
aqueous solutions and precipitate from solution. The resulting
solids can be isolated and their melting points determined.
The procedure you will use in this exercise exploits the
difference in acidity and solubility just described.
- (a) you will dissolve your unknown in ethyl acetate (an organic
solvent). All of the possible compounds are soluble in ethyl
acetate.
- (b) you will extract with sodium bicarbonate to remove any
carboxylic acid that is present.
- (c) you will extract with sodium hydroxide to remove any
phenol that is present.
- (d) you will acidify both of the resulting aqueous solutions to
cause any compounds that were extracted to precipitate.
These solids will be isolated by vacuum filtration, dried, and
then their melting point ranges determined to identify them. If a
neutral compound was present in your unknown, it will remain in
the organic layer throughout the extraction procedure. To isolate
it, you will simply evaporate the ethyl acetate to leave a solid.
The melting point ranges of all solids will be determined. You
will also weigh each solid you obtain to determine the percent
recovery of your procedure. Remember, though, that you only have
two compounds in your unknown mixture so that you should not
isolate solids from all of the extracts.
Extraction procedure:
General Notes: To measure the small volumes called for
in this procedure, it is convenient to measure them in a
graduated measuring cylinder. Make sure you label
everything so that you can identify which layer you are putting
into each flask correctly - label one 125 mL Erlenmeyer flask
"bicarbonate", a second one as "hydroxide", and a 50 mL
Erlenmeyer flask "ethyl acetate". We are using ethyl
acetate in this lab, so avoid excessive exposure. Be sure you are
familiar with the procedure below before starting the lab.
- Collect an unknown and record the unknown number. Without
this number, we cannot grade your report. Label three Erlenmeyer
flask as directed above.
- Dissolve approximately 1.0 g of your unknown mixture in 10 mL
of ethyl acetate.
- Pour the solution into a clean separatory funnel and add 10
mL of 10% aqueous sodium bicarbonate found on your bench.
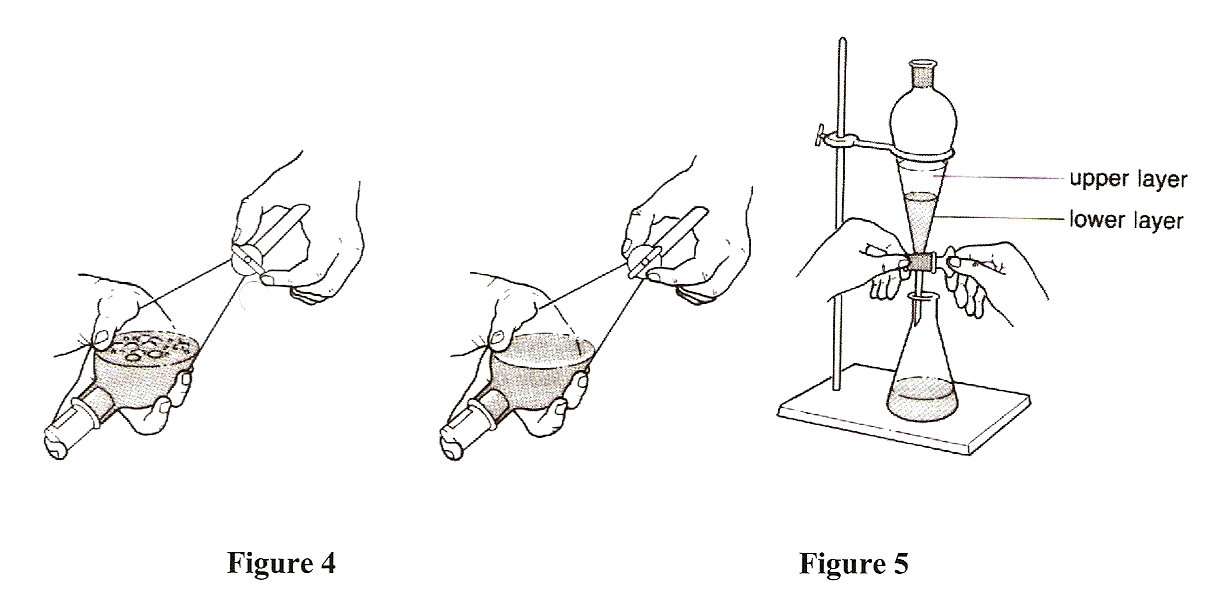
- Stopper the funnel and invert it. Slowly open the stopcock to
release any built up pressure, then close the stopcock
(Figure 4).
- Gently shake the separatory funnel to allow intimate mixing
of the solutions and effect extraction of the compound from the
organic mixture. (Caution: When shaken, the
mixture may develop pressure; be sure to vent it periodically).
- Clamp the separatory funnel to a retort stand and allow the
mixture to separate into two layers (Figure 5).
- Remove the stopper and collect the aqueous layer (the lower
layer) in the 125 mL Erlenmeyer flask labeled "bicarbonate".
- Repeat steps 3-7 two more times draining each portion
successively into the same flask. At the end of this sequence you
will have extracted the organic solution with three 10 mL
portions of 10% aqueous sodium bicarbonate.
- Put the Erlenmeyer flask labeled "bicarbonate" aside in a
safe place. Later you will isolate any compound that was
extracted by the bicarbonate. Do you remember which functional
group that would be?
- Add 10 mL of 5% aqueous NaOH to the separatory funnel with
the remaining ethyl acetate.
- Stopper the funnel and invert it. Slowly open the stopcock to
release any built up pressure, then close the stopcock.
- Gently shake the separatory funnel to allow intimate mixing
of the solutions and effect extraction of the compound from the
organic mixture.
- Clamp the separatory funnel to a retort stand and allow the
mixture to separate into two layers.
- Remove the stopper and collect the aqueous layer in the 125
mL Erlenmeyer flask labeled "hydroxide".
- Repeat steps 10-14 two more times draining each portion
successively into the same flask. At the end of this sequence you
will have extracted the organic solution with three 10 mL
portions of 5 % aqueous sodium hydroxide.
- Put the Erlenmeyer flask labeled "hydroxide" aside in a safe
place. Later, you will isolate any compound that was extracted by
the hydroxide. Do you remember which functional group that would
be?
The remaining steps described in this section will allow
you to isolate any compound remained in the ethyl acetate layer. Recall, this would be a
neutral compound, if you have one.
- Add 5 mL of saturated aqueous NaCl and 5 mL of distilled H2O
to the ethyl acetate layer in the separatory funnel.
- Separate and set aside the lower, aqueous layer.
- Pour the organic layer in the 50 mL Erlenmeyer flask and dry
with anhydrous Na2SO4.
- Filter the dried organic solution into a dry pre-weighed 50
mL round bottom flask and remove the ethyl acetate on a rotary
evaporator. If a solid remains after evaporation of the ethyl
acetate, it is a neutral substances and you will determine its
weight and melting point.
Instructions follow for isolating the carboxylic acid and / or
phenol from aqueous layers you put into the Erlenmeyer flasks
labeled "bicarbonate" and "hydroxide", respectively.
- Take the Erlenmeyer flask labeled "bicarbonate" and
carefully acidify the aqueous solution by the
dropwise addition of 6M HCl.
(CAUTION: The bicarbonate solution will
vigorously liberate carbon dioxide when neutralized with HCl -
that is, it will bubble a lot). Check to make sure the solution
is acidic with blue litmus paper.
- If a solid precipitates, add a boiling stone and then gently
heat the solution to bring most of the solid back into solution.
Cool slowly to room temperature and then use an ice/water bath to
complete the precipitation. If no solid precipitates, your
unknown did not contain a carboxylic acid. In that case, skip
steps 3-4.
- When the solution is ice cold, isolate the solid precipitate
by suction filtration.
- Filter, rinse the solid with ice-cold water, and determine
the weight and melting point range of the carboxylic
acid.
Now, we will follow the same procedure to isolate the phenol from
the Erlenmeyer flask labeled "hydroxide".
- Take the Erlenmeyer flask labeled "hydroxide" and
carefully acidify the aqueous solution in the
centrifuge tube by the dropwise
addition of 6M HCl. (CAUTION: The hydroxide
solution will become hot when neutralized with HCl). Check to
make sure the solution is acidic with blue litmus paper.
- If a solid precipitates, add a boiling stone and then gently
heat the solution to bring most of the solid back into solution. Cool slowly to room
temperature and then use an ice/water bath to complete the precipitation. If no solid
precipitates, your unknown did not contain a phenol. In that
case, skip steps 3-4.
- When the solution is ice cold, isolate the solid precipitate
by suction filtration.
- Filter, rinse the solid with ice cold water, and determine
the weight and melting point range of the phenol next week.
Safety Notes
You must wear eye protection at all times. In the event that
any reagent used in this investigation comes in contact with your
skin or eyes, wash the affected area immediately with lots of
water. Notify your instructor.
Avoid excessive exposure to all organic solvents.
Acids and bases can cause severe burns.
No flames should be present in the laboratory during this
experiment.
Acid/Base Extraction Flow chart
REFERENCES
L. M. Harwood and C. 1. Moody, Experimental Organic
Chemistry- Principles and Practice, Blackwell Scientific
Publications.
C.A. MacKenzie, Experimental Organic Chemistry,
Prentice-Hall. 4th Edition
J. A. Moore and D. L. Dalrymple, Experimental Methods in
Organic Chemistry, Saunders Golden Sunburst Series, W. B.
Saunders Company.
C. F. Wilcox and M. F. Wilcox, Experimental Organic
Chemistry- A Small-scale Approach, Prentice-Hall. 2nd
Edition.
O. R. Rodig, C. E. Bell Jr. and A. K. Clark, Organic
chemistry Laboratory- Standard and Microscale Experiments,
Saunders College Publishing.
J.R. Mohrig, C.N. Hammond and P.F. Schatz, Techniques in
Organic Chemistry, Freeman Publishers, 2nd Edition.
Copyright © 2009-2014 by The Department of Chemistry
UWI, Jamaica, all rights reserved.
Created and maintained by Prof. Robert J.
Lancashire,
The Department of Chemistry, University of the West Indies,
Mona Campus, Kingston 7, Jamaica.
Created Oct 2009. Links checked and/or last
modified 30th October 2014.
URL
http://wwwchem.uwimona.edu.jm/lab_manuals/c1901exp8.html