However, the role of the vitamin is not yet fully understood, although it is known that it is essential in preventing the disease pernicious anaemia.
Three biologically active cobalamins can be detected in the serum and tissues of man and higher animals, these are: adenosylcobalamin, AdoCbl; hydroxocobalamin, OH-Cbl; and methyl-cobalamin, MeCbl. Cyanocobalamin, CN-Cbl has been detected in chromatograms of the blood of smokers, where it is believed to be formed from HCN from cigarettes reacting with OH-Cbl. It has no physiological role (apart from this unexpected detoxification of cyanide) although it has been used for treating pernicious anaemia because like OH-Cbl, it can be converted in vivo into Ado-Cbl and MeCbl.
Pernicious anaemia is largely a disease affecting people over the age of 40
and is almost unknown in negroes. In the UK, it occurs with a frequency
of 1 in 1000. The consequence of depriving people of essential
cobalamins is debilitation which if continued can end in death.
The model compound to be prepared in this experiment contains an
ethyl-cobalt bond. The cobaloximes have been used as models due to
their ease of preparation, purification and characterisation.

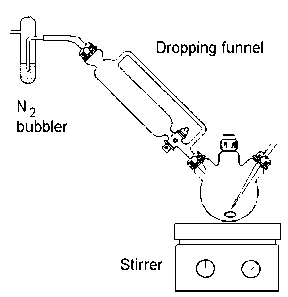
Carefully remove the stopper briefly (increase the gas flow momentarily)
then dimethylglyoxime (2.32 g, 20 mmol) is added, followed by finely
powdered cobalt(II) chloride hexahydrate (2.37 g, 10 mmol). Not all the
reactants will dissolve but the deep brown colouration due to the Co(II)
complex should appear. Sodium hydroxide (1.6 g, 40 mmol) dissolved in the
minimum amount of water (about 5 cm3) is added via the dropping funnel.
The intense blue-black colour of the cobalt(I) intermediate should
develop over the next 2-4 minutes. If it does not then air has attacked
the cobalt(I) intermediate and the experiment should be repeated (see
the demonstrator first).
Next, a solution of iodoethane (0.78 g, 5 mmol) dissolved in a little
methanol (about 5 cm3) is added, again via the dropping funnel. The
organocobaloxime forms immediately as evidenced by the blue suspension
turning brown. The methanol solvent is removed by rotary evaporation at
about 50 óC(higher temperatures may lead to decomposition). The pasty
mass is stirred with a slush of ice and water (about 200 cm3). This
oxygenates and solubilises the unwanted by-products but does not
significantly decrease the yield of product.
The orange brown cobalt complex is filtered on a sintered glass funnel
and washed with 100 cm3 of ice-cold water. Dry in a dessicator in the
dark (the product is somewhat light sensitive) and record the yield.
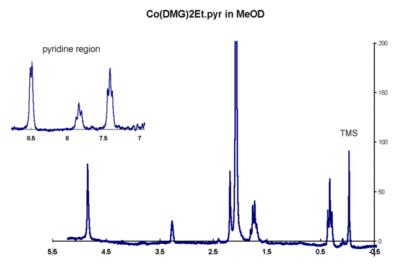 NMR
spectrum in JCAMP-DX format
NMR
spectrum in JCAMP-DX format
Account for this and the appearance of the other peaks in the spectrum.
An IR spectrum is available as well. Identify as many peaks as you can.
 Return to Chemistry,
UWI-Mona, Home Page
Return to Chemistry,
UWI-Mona, Home Page
Nov-96, rjl