Preparation of a Nickel(II) tetraazamacrocycle.
Introduction
The first and most extensively studied series of synthetic tetraaza macrocycles
were initially prepared by Curtis, who reported that the reaction of
tris-(1,2-diaminoethane)nickel(II) perchlorate and acetone at room temperature
produced a yellow crystalline product. The compound was diamagnetic
and very resistant to hydrolysis. The original structure proposed was
not a macrocycle but this was later corrected to fit in with the
chemical inertness, the infrared spectrum
and its reaction with hydrogen to give 1,2-diaminoethane and mesityl oxide.
The product can occur in two noninterconvertible forms, which Curtis originally
designated as A and B. The A isomer occurring in two interconvertible
forms called Aa and Ab.
The three isomers are similar in appearance and physical properties although
some differences in their IR spectra have been noted. The first crystals of
cyclic complex which crystallise from the reaction medium are
isometrically pure B(ClO4)2.
The Aa(ClO4)2 isomer usually begins to crystallise after about 25% of
the total product has separated.
The separation occurs since the B form is less soluble. The A-B
isomerism comes from
cis- trans arrangements of the imine linkages in the macrocyclic ring,
with A corresponding to the trans- form and B the cis-isomer.
Later studies have shown that Aa is the more stable form and that it
corresponds to the racemic mixture of isomers (the asymmetry exists at
the tetrahedral nitrogen atoms in the diene complex) and that Ab is the
meso isomer.
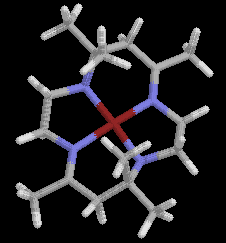 Isomer B is
also a racemate.
Isomer B is
also a racemate.
Procedure
Preparation of [Ni(en)3]Cl2
70% aqueous 1,2-diaminoethane (ethylenediamine, 2.8 g, 3.1 cm3) is added to
a solution of nickel(II) chloride hexahydrate (2.4 g) in 10 -15 cm3 of water.
The resulting purple solution is filtered to remove any insoluble
material and the solution is then evaporated down to approximately 6 cm3
on a steam bath. Two drops of 1,2 diaminoethane
are then added and the solution is cooled in an ice-bath.
The orchid coloured crystals are collected on a sintered glass filter funnel
and washed twice with 3 cm3 portions of ethanol and finally air-dried.
The mother liquor should not be discarded since further product can be obtained
by addition of ethanol and further cooling.
Record the yield and show the product to a demonstrator.
Conversion to the perchlorate salt.
The perchlorate salt is more soluble in acetone than the chloride and
this is used for the subsequent reaction.
Tris-(1,2-diaminoethane)nickel(II) chloride (2 g) is dissolved in hot ethanol
and sodium perchlorate (2 g) is then added. The solution is cooled and
the complex precipitating out is collected by suction.
Record the yield and show the product to a demonstrator.
NB. Perchlorate salts have been known to detonate spontaneously
and should be treated as potentially hazardous.
Preparation of the Macrocyclic complex.
Tris-(1,2-diaminoethane)nickel(II) perchlorate dihydrate (0.5 g) is dissolved
in 7 cm3 of acetone and the purple solution is thoroughly shaken. The
reaction mixture is left to stand for a few hours (may be left
overnight) after which yellow crystals are formed.
These are collected by suction and air-dried.
Record the yield and show the product to a demonstrator.
An IR spectrum is available, record
the major peaks and try to account for their position and intensity.
Questions
1. Outline a possible reaction scheme for the template synthesis of the
Ni(II) complex.
2. What is The Macrocyclic Effect?
References
L.F. Lindoy, "The Chemistry of Macrocyclic Ligand Complexes", Cambridge
University Press, 1989, Chapter 2.
G.A. Melson, "Coordination Chemistry of Macrocyclic Compounds", Plenum
Press, New York, 1979. Chapter 2.
return to list of C31L
experiments
 Return to Chemistry,
UWI-Mona, Home Page
Return to Chemistry,
UWI-Mona, Home Page
Nov-96, rjl
 Isomer B is
also a racemate.
Isomer B is
also a racemate. Return to Chemistry,
UWI-Mona, Home Page
Return to Chemistry,
UWI-Mona, Home Page