CHEM1902 Main Group Chemistry
Tutorial 3 - 2015
Prof Robert J. Lancashire
Muliple Choice Questions
1. In which of the following reactions is H2O2 not acting as an oxidizing agent?
a) 2Fe2+ + H2O2 + 2H+ → 2Fe3+ + 2H2O
b) PbS + 4H2O2 → PbSO4 + 4H2O
c) 2I- + 2H2O2 + 2H+ → I2 + 2H2O
d) Mn2+ + H2O2 + 2OH- → MnO2 + 2H2O
e) Ag2O + 2H2O2 → 2Ag + O2 + H2O
Short Answer Questions
- You are provided with two liquids, one is H2O and the other
is H2O2. Safety regulations now prohibit you from
touching/tasting them so suggest chemical tests that could distinguish between
them. Include the reagents and observations expected and show balanced
equations for the reactions.
-
Use the MO diagram below to determine the bond order for the following species
and predict whether the bond lengths shown are consistent with your values.
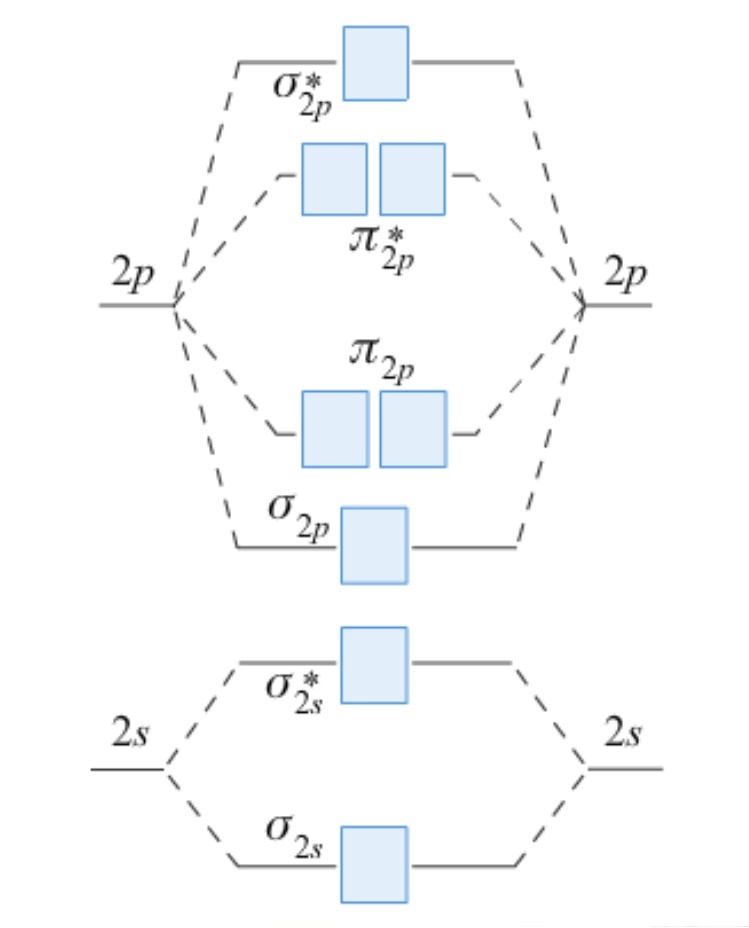
|
O2+ = 112 pm
O2 = 121 pm
O2- = 128 pm
O22- = 149 pm
|
- With the aid of suitable equations describe how H2O2
can be produced industrially and on a small scale in the laboratory.
- Write balanced half equations and the net ionic equation for:
i) the oxidation of Fe2+ by H2O2 in acidic solution.
ii) the reaction between KMnO4 and H2O2 in acidic solution.
iii) the reaction between acidified KBr and H2O2.
- Describe two uses of D2O, including the chief large scale use.
- With the aid of equations, describe how ozone is produced and destroyed
in the upper atmosphere.
- How would you prepare ozone in the laboratory? What conditions and
safety equipment would be needed?
- What experiment/techniques could be used to distinguish between O3
and O2?
- How does the O-O bond length in O3 of 127.2 pm compare to the
data above for O2 species?
return to the course outline
 Return to Chemistry, UWI-Mona,
Home Page
Return to Chemistry, UWI-Mona,
Home Page
Copyright © 2015 by Robert John
Lancashire, all rights reserved.
Created and maintained by Prof. Robert J.
Lancashire,
The Department of Chemistry, University of the West Indies,
Mona Campus, Kingston 7, Jamaica.
Created November 2014. Links checked and/or last
modified 16th February 2015.
URL
http://wwwchem.uwimona.edu.jm/tutorials/ICHEM1902_MGTut3.html


 Return to Chemistry, UWI-Mona,
Home Page
Return to Chemistry, UWI-Mona,
Home Page