Chemistry of Titanium
An excellent site for finding the properties of the elements,
including Titanium is at 
For the chapter on Titanium chemistry from the Elsevier text
"Chemistry of the Elements" by Greenwood and Earnshaw see
On-Line Metals Based Surveys
History
The discovery of titanium in 1791 is attributed to William Gregor, a Cornish vicar
and amateur chemist. He isolated an impure oxide from ilmenite (FeTiO3)
by treatment with HCl and H2SO4.
Introduction
Titanium is the second most abundant transition metal on Earth (6320 ppm) and
plays a vital role as a material of construction because of its:
- Excellent Corrosion Resistance
- High Heat Transfer Efficiency
- Superior Strength-To-Weight Ratio
For example, when it's alloyed with 6% aluminum and 4% vanadium,
titanium has half the weight of steel and up
to four times the strength.
Whilst a biological function in man is not known, it has excellent
biocompatibility--that is the ability to be ignored by the human
body's immune system--and an extreme resistance to
corrosion. Titanium is now the metal of choice for hip and
knee replacements.
Occurrence
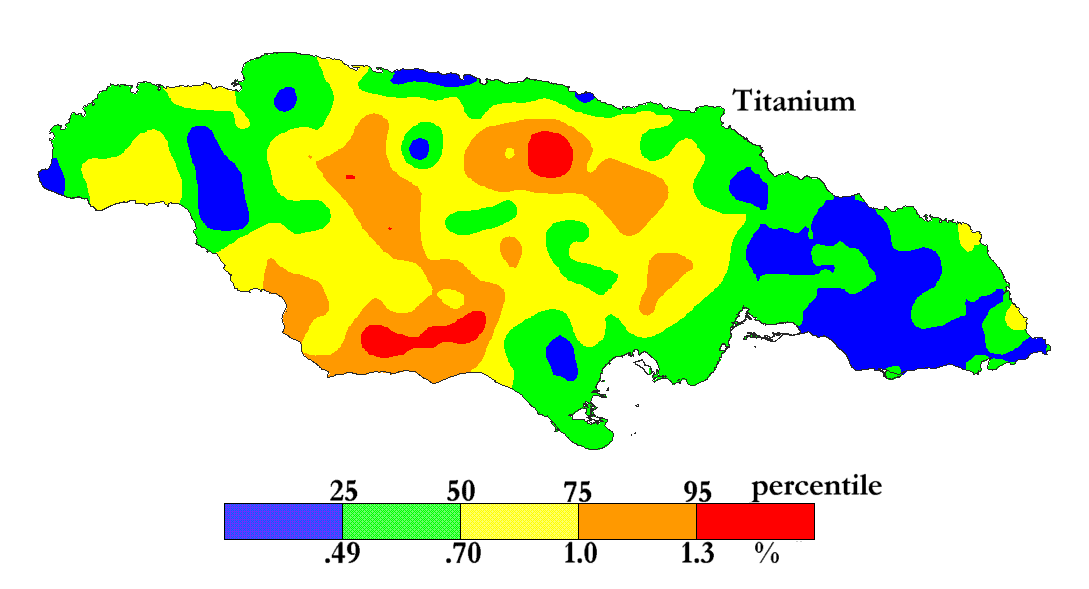
The International Centre for Environmental and Nuclear Sciences (ICENS)
has an on-going programme of mapping the geochemical content of Jamaica.
'A Geochemical Atlas of Jamaica' was published in 1995 and is available from Amazon or ICENS.
The results found for Titanium are shown below (courtesy of Prof G.C. Lalor).
Properties of titanium
An excellent site for finding the properties of the elements,
including titanium is at 
Extraction of Titanium - the Kroll process
Wilhelm J. Kroll
Born November 24, 1889 - Died March 30, 1973
He developed the process in Luxemburg around the mid 1930's
and then after moving to the USA extended it to enable the
extraction of Zirconium as well.
Titanium ores, mainly rutile (TiO2) and ilmentite
(FeTiO3), are treated with carbon and chlorine gas to
produce titanium tetrachloride.
TiO2 + Cl2 ->TiCl4 +
CO2
Fractionation
Titanium tetrachloride is purified by distillation (BP 136.4) to remove
iron chloride.
Reduction
Purified titanium tetrachloride is reacted with molten magnesium
under argon to produce a porous “titanium
sponge”.
TiCl4 + 2Mg -> Ti + 2MgCl2
Melting
Titanium sponge is melted under argon to produce ingots.
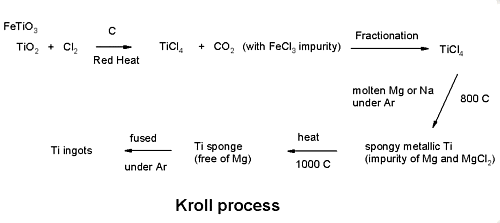 The Kroll process (ISIS Draw .skc file)
The Kroll process (ISIS Draw .skc file)
Titanium Halides
Titanium(IV) Halides
| Formula |
Colour |
MP |
BP |
Structure |
| TiF4 |
white |
- |
284 |
fluoride bridged |
| TiCl4 |
colourless |
-24 |
136.4 |
- |
| TiBr4 |
yellow |
38 |
233.5 |
hcp I- but essentially monomeric cf. SnI4 |
| TiI4 |
violet-black |
155 |
377 |
hcp I- but essentially monomeric cf. SnI4 |
Preparations:
They can all be prepared by direct reaction of Ti with halogen
gas (X2). All are readily hydrolysed.
They are all expected to be diamagnetic.
Titanium(III) halides
| Formula |
Colour |
MP |
BP |
m (BM) |
Structure |
| TiF3 |
blue |
950d |
- |
1.75 |
- |
| TiCl3 |
violet |
450d |
- |
- |
BiI3 |
| TiBr3 |
violet |
- |
- |
- |
BiI3 |
| TiI3 |
violet-black |
- |
- |
- |
- |
Preparations:
They can be prepared by reduction of TiX4 with
H2.
Titanium Oxides and Aqueous Chemistry
Titanium oxides
| Formula |
Colour |
MP |
m (BM) |
Structure |
| TiO2 |
white |
1892 |
diam. |
rutile - Refractive Index 2.61-2.90
cf. Diamond 2.42 |
Preparations:
obtained from hydrolysis of TiX4 or Ti(III)
salts.
TiO2 reacts with acids and bases.
In Acid:
TiOSO4 formed in H2SO4 (Titanyl
sulfate)
In Base:
MTiO3 metatitanates (eg Perovskite, CaTiO3
and ilmenite, FeTiO3)
M2TiO4 orthotitanates.
Peroxides are highly coloured and can be used for
colourimetric analysis.
pH <1 [TiO2(OH)(H2O)x]+
pH 1-2 [(O2)Ti-O-Ti(O2)](OH)
x2-x; x=1-6
[Ti(H2O)6]3+ ->
[Ti(OH)(H2O)5]2+ + [H+]
pK=1.4
TiO2+ + 2H+ + e- -> Ti3+ +
H2O E=0.1V
Representative complexes
TiCl4 is a good Lewis acid and forms adducts on
reaction with Lewis bases such as;
2PEt3 -> TiCl4(PEt3)2
2MeCN -> TiCl4(MeCN)2
bipy -> TiCl4(bipy)
Solvolysis can occur if ionisable protons are present in the
ligand;
2NH3 -> TiCl2(NH2)2 + 2HCl
4H2O -> TiO2.aq + 4HCl
2EtOH -> TiCl2(OEt)2 + 2HCl
TiCl3 has less Lewis acid strength but can form
adducts also;
3pyr -> TiCl3pyr3
References:
"Complexes and First-Row Transition Elements", D. Nicholls
"Basic Inorganic Chemistry", F.A. Cotton, G. Wilkinson and P.L. Gaus
"Advanced Inorganic Chemistry", F.A. Cotton, G. Wilkinson, C. A. Murillo, and M. Bochmann
"Chemistry of the Elements", Greenwood and Earnshaw
return to the C21J course
outline
 Return to Chemistry, UWI-Mona,
Home Page
Return to Chemistry, UWI-Mona,
Home Page
Copyright © 2001-2007 by Robert John
Lancashire, all rights reserved.
Created and maintained by Prof. Robert J.
Lancashire,
The Department of Chemistry, University of the West Indies,
Mona Campus, Kingston 7, Jamaica.
Created October 2001. Links checked and/or
last modified 14th November 2007.
URL
http://wwwchem.uwimona.edu.jm/courses/titanium.html

 The Kroll process (ISIS Draw .skc file)
The Kroll process (ISIS Draw .skc file) Return to Chemistry, UWI-Mona,
Home Page
Return to Chemistry, UWI-Mona,
Home Page