Chemistry of Vanadium
For the chapter on Vanadium chemistry from the Elsevier text
"Chemistry of the Elements" by Greenwood and Earnshaw see
On-Line Metals Based Surveys
History
The discovery of vanadium is attributed to Andres Manuel del Rio
(a Spanish mineralogist working in Mexico City) who prepared a
number of salts from a material contained in "brown lead" around 1801.
Unfortunately, the French chemist Collett-Desotils incorrectly
declared that del Rio's new element was only impure Chromium. Del
Rio thought himself to be mistaken and withdrew his claim.
The element was rediscovered in 1830 by the Swedish chemist Nils
Gabriel Sefström who named it after the Norse goddess
Vanadis, the goddess of beauty and fertility.
Metallic vanadium was not isolated until 1867 when
Sir Henry Enfield Roscoe (1833-1915), Professor of Chemistry at Owens College
(later the University of Manchester) from 1857 to 1885, reduced
vanadium chloride (VCl5) with gaseous hydrogen to give vanadium metal and HCl.
Occurrence
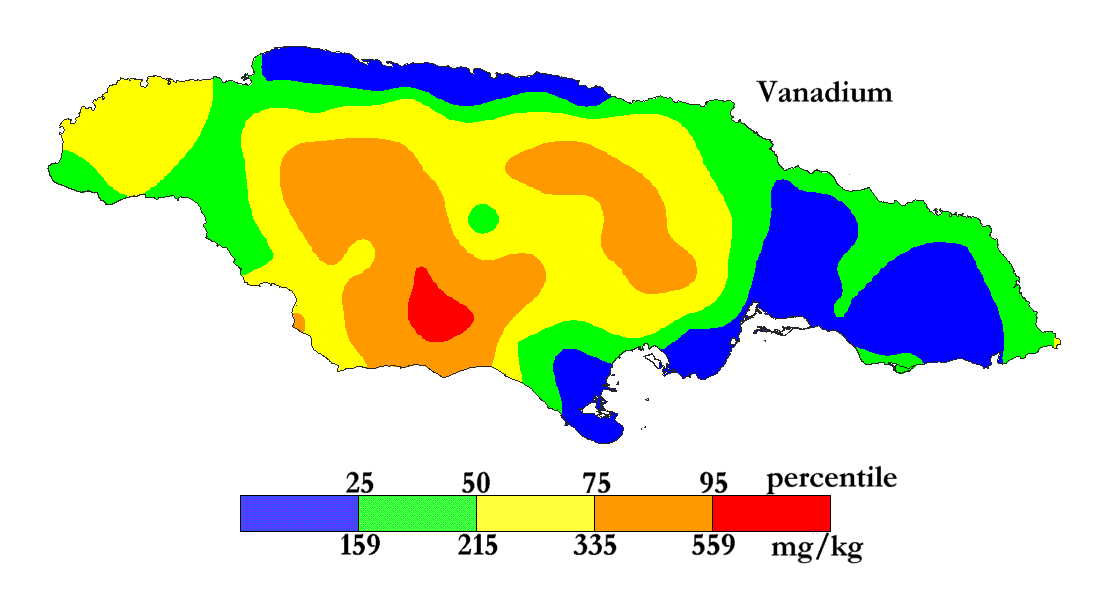
The International Centre for Environmental and Nuclear Sciences (ICENS)
has an on-going programme of mapping the geochemical content of Jamaica.
'A Geochemical Atlas of Jamaica' was published in 1995 and is available from Amazon or ICENS.
The results found for Vanadium are shown below (courtesy of Prof G.C. Lalor).
Properties of vanadium
An excellent site for finding the properties of the elements,
including vanadium is at 
Introduction
Vanadium has been found to play a number of roles in biological
systems. It is present in certain vanadium dependent
haloperoxidase and nitrogenase enzymes.

A tunicate (
Clavelina Puertosecensis) discovered near Discovery Bay, Jamaica
Many sea squirts, such as
Ciona Intestinalis accumulate vanadium in very high concentration, although the
reason is not known.
The mushroom Amanita muscaria
accumulate vanadium in the form of a coordination complex called
amavadin, whose function is still unknown.
A number of vanadium complexes have been shown to alleviate many of the
symptoms of diabetes in both in vitro and in vivo (in rats and
mice) studies. These complexes are being studied as potential
alternatives to insulin therapy.
Vanadium Halides
Vanadium(V) halides
| Formula |
Colour |
MP |
BP |
m (BM) |
Structure |
| VF5 |
white |
19.5 |
48.3 |
0 |
trigonal bipyramid in gas phase |
Preparations:
Prepared by reaction of V with F2 in N2
or with BrF3 at 300C.
In the solid state it is an infinite
chain polymer with cis-fluoride bridging.
Vanadium(IV) halides
| Formula |
Colour |
MP |
BP |
μ (BM) |
Structure |
| VF4 |
lime-green |
100 (a) |
- |
1.68 |
- |
| VCl4 |
red-brown |
-25.7 |
148 |
1.61 |
tetrahedral (monomeric) |
| VBr4 |
purple |
-23d |
- |
- |
- |
(a) sublimes with decomposition at 100 C.
Preparations:
VCl4 is prepared by reaction of V with chlorinating
agents such as Cl2, SOCl2, COCl2
etc.
Reaction of VCl4 with HF in CCl3F at -78C
gives VF4.
Vanadium Oxides and Aqueous Chemistry
Vanadium oxides
| Formula |
Colour |
Common name |
Oxidation State |
MP |
V-O distance (pm) |
| V2O5 |
brick-red |
pentoxide |
V5+ |
658 |
158.5-202 |
| V2O4 |
blue |
dioxide |
V4+ |
1637 |
176-205 |
| V2O3 |
grey-black |
sesquioxide |
V3+ |
1967 |
196-206 |
Preparations:
V2O5 is the final product of the oxidation
of V metal, lower oxides etc.
Aqueous Chemistry very complex:
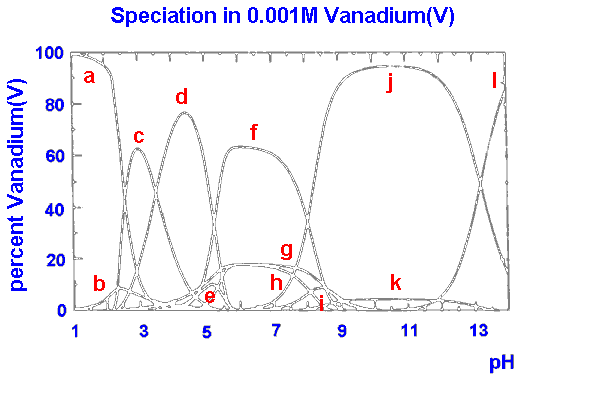
|
a VO2+
b VO(OH)3
c V10O26(OH)24-
d V10O27(OH)5-
e V10O286-
f V3O93-
g VO2(OH)2-
h V4O124-
i V2O6(OH)3-
j VO3(OH)2-
k V2O74-
l VO43-
|
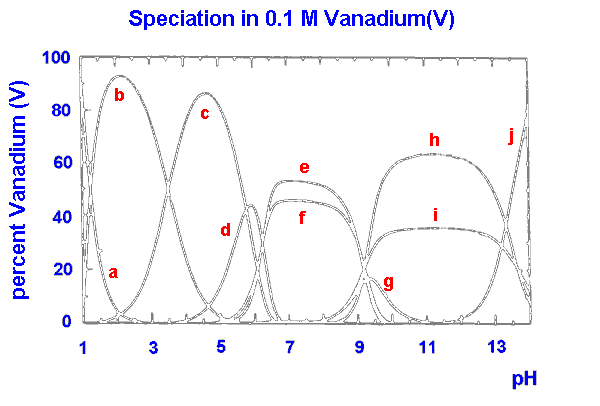
|
a VO2+
b V10O26(OH)24-
c V10O27(OH)5-
d V10O286-
e V4O124-
f V3O93-
g V2O6(OH)3-
h V2O74-
i VO3(OH)2-
j VO43-
|
In alkaline solution,
VO43- + H+ →
HVO42-
2HVO42- →
V2O74- + H2O
HVO42- + H+ →
H2VO4-
3H2VO4- →
V3O93- + 3H2O
4H2VO4- →
V3O124- + 4H2O
In acidic solution,
10V3O93- +
15H+ → 3HV10O285-
+ 6H2O
H2VO4- + H+ →
H2VO4
HV10O285- + H+ →
H2V10O284-
H3VO4 + H+ →
VO2+ + 2H2O
H2V10O284- +
14H+ → 10VO2+ +
8H2O
VO(H2O)4SO4
The crystal structure of this salt was first determined in 1965.
The V=O bond length was 159.4 pm, the aquo group trans to this
had the longest V-O bond length (228.4pm) and the equatorial bond
lengths were in the range 200.5-205.6 pm. Note that
SO42- was coordinated in an equatorial
position.
The IR stretching frequency for the V=O in vanadyl complexes
generally occurs at 985 +/- 50 cm-1.
Redox properties of oxovanadium ions:
VO2+ + 2H+ + e-
→ VO2+ + H2O E=1.0 V
VO2+ + 2H+ + e- → V3+ +
H2O E=0.34 V
References:
"Inorganic Chemistry", 3rd Edition, Catherine Housecroft, Alan G. Sharpe, Publisher: Prentice Hall
"Complexes and First-Row Transition Elements", D. Nicholls
"Basic Inorganic Chemistry", F.A. Cotton, G. Wilkinson and P.L. Gaus
"Advanced Inorganic Chemistry", F.A. Cotton, G. Wilkinson, C. A. Murillo, and M. Bochmann
"Chemistry of the Elements", Greenwood and Earnshaw
"Hydrolysis of Cations", Baes and Messmer
return to the CHEM2101 (C21J) course
outline
 Return to Chemistry, UWI-Mona,
Home Page
Return to Chemistry, UWI-Mona,
Home Page
Copyright © 2001-2011 by Robert John
Lancashire, all rights reserved.
Created and maintained by Prof. Robert J.
Lancashire,
The Department of Chemistry, University of the West Indies,
Mona Campus, Kingston 7, Jamaica.
Created September 2001. Links checked and/or
last modified 1st April 2011.
URL
http://wwwchem.uwimona.edu.jm/courses/vanadium.html





 Return to Chemistry, UWI-Mona,
Home Page
Return to Chemistry, UWI-Mona,
Home Page