EXPERIMENT 10
Preparation of cyclohexene from cyclohexanol.
Aim:
The objective of this exercise is to prepare cyclohexene from
cyclohexanol and determine the efficiency of this conversion.
Experimental learning objectives: How to;
- set up a distillation apparatus and perform a
distillation
- use a separatory/dropping funnel
- wash and dry an organic liquid
- identify the organic phase in an immiscible organic/aqueous
mixture
- synthesise an alkene by dehydration of an alcohol
- identify the presence of unsaturation in an organic molecule
using both chemical reactions and IR spectroscopy
In this experiment an alkene (cyclohexene) will be prepared by
dehydration of an alcohol (cyclohexanol) using an acid catalyst
such as phosphoric acid. This is one of the most common methods
of preparing alkenes.
The crude product is contaminated with water, unreacted alcohol,
phosphoric acid and some side products. Washing with water
removes most of the impurities. Treatment with sodium carbonate
solution removes traces of acid and a final wash with water
removes any remaining carbonate.
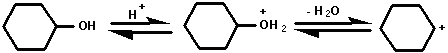
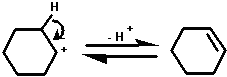
The mechanism of the dehydration of cyclohexanol probably
involves the formation of a carbocation.
This carbocation can react in any of the ways shown below:
1. With water to yield cyclohexanol - the starting
material. (Note that all the steps in this reaction are
reversible);
2. by losing a proton to yield cyclohexene;
3. with cyclohexanol to yield dicyclohexyl ether.
Dicyclohexyl ether then is a probable side product of the
dehydration of cyclohexanol. It is immiscible with water is
likely to co-distill and may therefore be present in the first
distillate. To remove dicylcohexy ether completely a second
distillation of the product is usually carried out.
Reference:
T.W.G. Solomons and C. Fryhle, Organic Chemistry, Chapter 7.7,
Dehydration of Alcohols.
Safety Features - CAUTION!
1. You must wear eye protection at all times.
2. Phosphoric acid (85%) can cause severe burns. Wash all spills
on the skin with cold water for 15 min. See your
demonstrator.
3. Bromine causes severe burns. Keep bromine away from your skin.
Do not breathe the vapours. Use only in the FUME HOOD.
4. Cyclohexanol can be irritating to the respiratory system and
skin. Do not breathe vapours and prevent contact with skin.
5. Potassium permanganate is a strong oxidizing agent. Handle
with care.
6. Cyclohexene has an unpleasant smell. Cover all containers of
this compound and do not leave drying agent, glass wool or towels
coated with the compound lying on the bench.
Procedure:
Pour cyclohexanol (10.0 g, 10.6 mL, b.p. 161°) into a 50 mL
round bottom flask (small neck) and cautiously add 85% phosphoric
acid (3 mL). Add 3 boiling chips and arrange for a distillation
using a cooled 10 mL graduated cylinder as a receiver. (Cool the
cylinder by standing it in a beaker of ice and water).
Heat the round bottom flask slowly with a small flame. When white
fumes appear in the round bottom flask, and
about 10 mL of distillate have been collected, discontinue the
distillation.
Transfer the distillate to a separatory funnel (60 mL) (Check
first to ensure that the stopcock of the separatory flask is
closed!). The mixture will separate into two layers.
Run off the lower layer into a conical flask and set this
aside.
Add water (10mL) to the liquid in the separatory funnel, stopper
the funnel and shake to allow thorough mixing of the liquids.
(This exercise is termed "washing the cyclohexene with
water".
Return the funnel to the clamp, loosen the stopper and allow the
layers to separate. Cyclohexene (density 0.81 g cm-3)
will separate out from the water. Run off the aqueous layer.
(Make sure that it is aqueous by adding a drop of water to the
flask in which you have collected it!!)
Now wash the cyclohexene (where is it?) with 10% sodium carbonate
solution (10 mL). Allow the layers to separat and run off the
aqueous layer.
Repeat the washing with water (10 mL).
Transfer the cyclohexene into a clean dry conical flask and add a
spatula full of calcium chloride. (Please remember to quickly
cover the bottle of calcium carbonate after use since it is
hygroscopic and will soon pick up moisture rendering it useless
for the rest of the class).
Stopper your conical flask, swirl the mixture and allow to stand.
If the mixture is cloudy you will need to add another spatula
full of calcium carbonate. Allow the solution to stand over the
drying agent for about fifteen minutes, then, by gravity
filtration using cotton wool, filter the cyclohexene into a dried
pre-weighed test-tube.
Immediately stopper the tube with a cork and discard the drying
agent and cotton wool into the waste containers which are in the
fume hood.
If the liquid is cloudy indicating the presence
of moisture, add a few lumps of calcium chloride to the test tube
and allow the mixture to stand until clear. Decant the liquid to
a clean, dry pre-weighed vial that has been labelled in the usual
way.
If the liquid is clear, place the sample in a
suitably labelled vial. Calculate the determine the yield and
percentage yield of cyclohexene. Carry out the
bromine/dichloromethane and permanganate tests (see Appendix I on
Tests for functional groups) on your product. Have the remainder
of your sample available for grading.
Note 1. Filtration by gravity will occur much more readily if you place
a paper wedge between your funnel and your test tube. Be careful not to
let the wedge fall into your solution on removing the funnel.
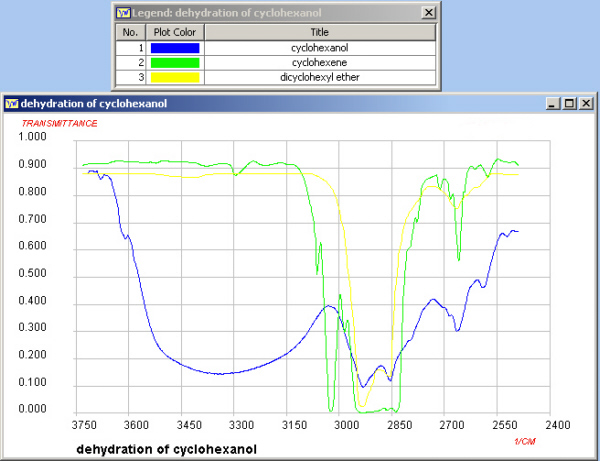
Infrared Spectroscopy
In this experiment an alkene has been prepared from an alcohol.
Functional group absorptions can be used to assist in the
identification of reactants and products. (See Appendix 2). The region shown below is
where the O-H and C-H stretches are found and if you ran a sample
of your own product it should be possible to determine whether
the conversion was successful.
Note on your worksheet the value of the O-H absorption of
cyclohexanol and the value of the C=C absorption of
cyclohexene.
An
interactive display of the IR of several C6 species is available, including
cyclohexene and cyclohexanol.
 Return to Chemistry, UWI-Mona,
Home Page
Return to Chemistry, UWI-Mona,
Home Page
Copyright © 2002-2014 by The Department
of Chemistry UWI, Jamaica, all rights reserved.
Created and maintained by Prof. Robert J.
Lancashire,
The Department of Chemistry, University of the West Indies,
Mona Campus, Kingston 7, Jamaica.
Created Oct 2002. Links checked and/or last
modified 16th October 2014.
URL
http://wwwchem.uwimona.edu.jm/lab_manuals/c10expt10.html
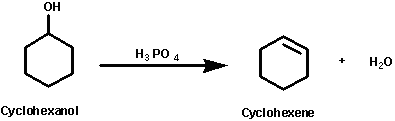
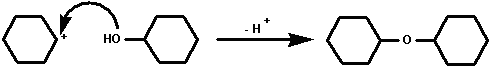
 Return to Chemistry, UWI-Mona,
Home Page
Return to Chemistry, UWI-Mona,
Home Page