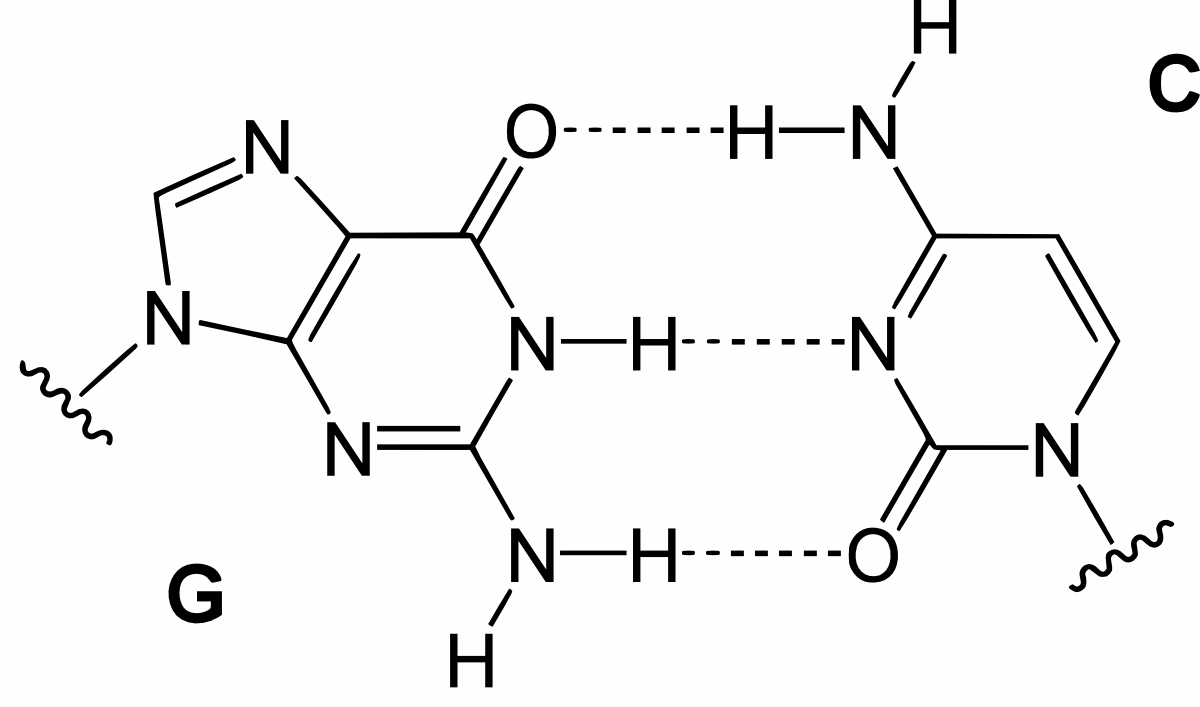
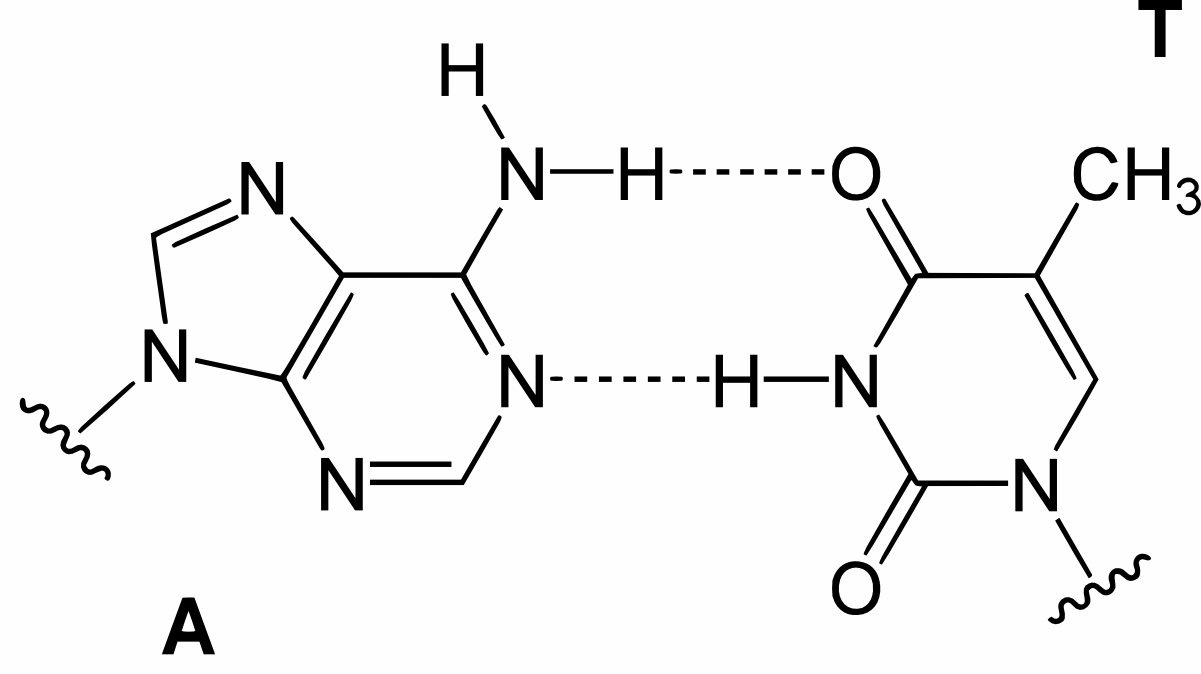
Hydrogen bonding in base pairs
|
|
|
|
Applications of hydrogen
Large quantities of H
2 are used by the petroleum and chemical industries.
The largest application of H
2 is for the processing ("upgrading") of fossil
fuels, and in the production of ammonia. The key consumers of H
2 in the
petrochemical plant include hydrodealkylation, hydrodesulfurization, and
hydrocracking. H
2 has several other important uses. H
2 is used as a
hydrogenating agent, particularly in increasing the level of saturation of
unsaturated fats and oils (found in items such as margarine), and in the
production of methanol. It is similarly the source of hydrogen in the
manufacture of hydrochloric acid. H
2 is used as a reducing agent of
metallic ores.
Nitrogen is a strong limiting nutrient in plant growth. Carbon and oxygen
are also critical, but are more easily obtained by plants from soil and air.
Even though air is 78% nitrogen, atmospheric nitrogen is nutritionally
unavailable because nitrogen molecules are held together by strong triple
bonds. Nitrogen must be 'fixed', i.e. converted into some bioavailable form,
through natural or man-made processes. It was not until the early 20th century
that Fritz Haber developed the first practical process to convert atmospheric
nitrogen to ammonia, which is nutritionally available.
Fertilizer generated from ammonia produced by the
Haber process is estimated
to be responsible for sustaining one-third of the Earth's population.
It is estimated that half of the protein within human beings is made of
nitrogen that was originally fixed by this process; the remainder was produced
by nitrogen fixing
bacteria and archaea.
Dozens of chemical plants worldwide produce
ammonia, consuming more than
1% of all man-made power. Ammonia production is thus a significant component
of the world energy budget. Modern ammonia-producing plants depend on industrial
hydrogen production to react with atmospheric nitrogen using a magnetite
catalyst or over a promoted Fe catalyst under high pressure (100 standard
atmospheres (10,000 kPa)) and temperature (450 °C) to form anhydrous liquid
ammonia. This step is known as the ammonia synthesis loop (also referred to
as the
Haber-Bosch process):
3 H2 + N2 ⇄ 2 NH3 (ΔH = -92.4 kJmol-1)
Nitrogen (N
2) is very unreactive because the molecules are held
together by strong triple bonds. The Haber process relies on catalysts that
accelerate the cleavage of this triple bond.
At room temperature, the equilibrium is strongly in favor of ammonia,
but the reaction doesn't proceed at a detectable rate. Thus two
opposing considerations are relevant to this synthesis.
One possible solution is to raise the temperature, but because the reaction
is exothermic, the equilibrium quickly becomes quite unfavourable at
atmospheric pressure. Low temperatures are not an option since the catalyst
requires a temperature of at least 400 °C to be efficient.
By increasing the pressure to around 200 atm the equilibrium
concentrations are altered to give a profitable yield.
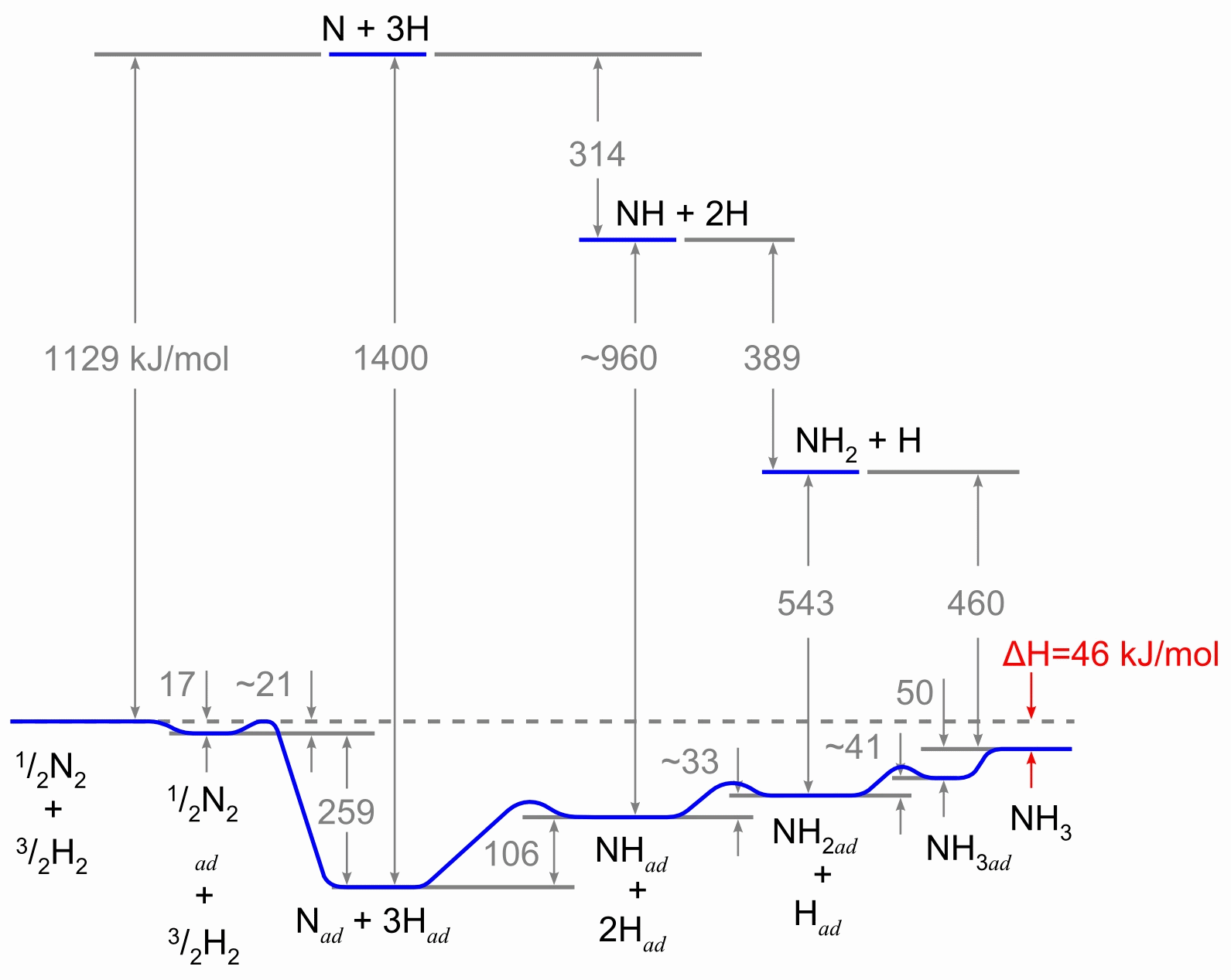
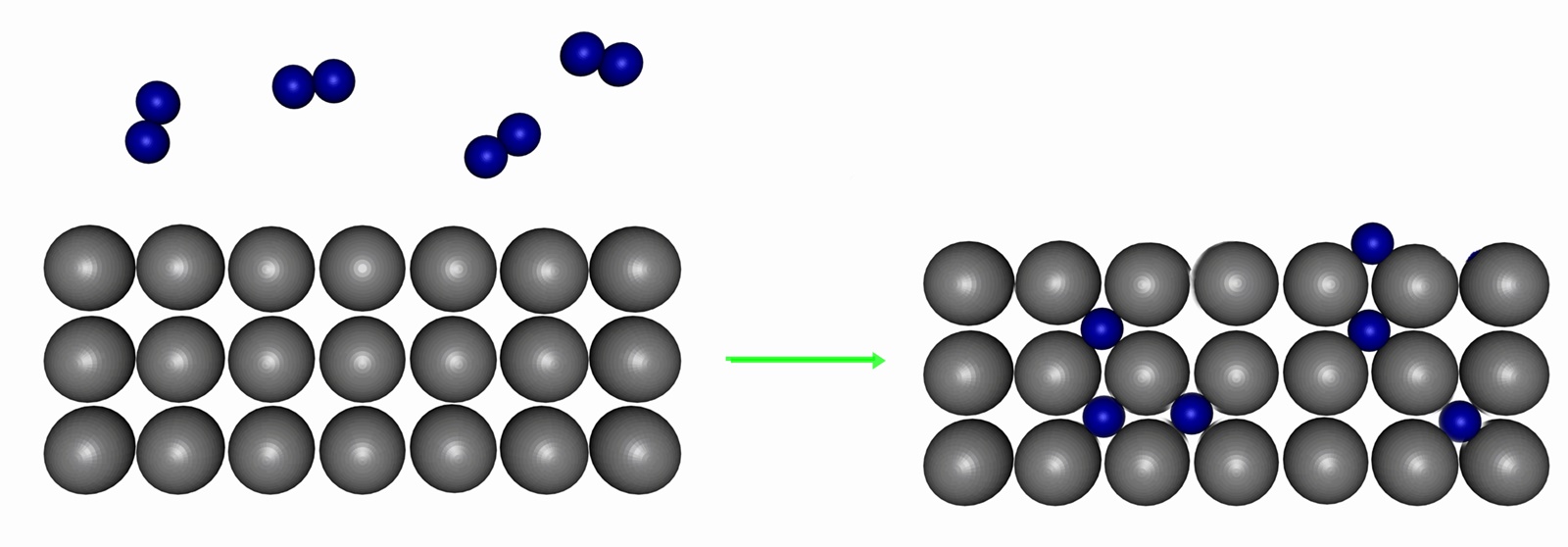
The reaction scheme, involving the heterogeneous catalyst, is believed to
involve the following steps:
- N2 (g) → N2 (adsorbed)
- N2 (adsorbed) → 2 N (adsorbed)
- H2(g) → H2 (adsorbed)
- H2 (adsorbed) → 2 H (adsorbed)
- N (adsorbed) + 3 H (adsorbed) → NH3 (adsorbed)
- NH3 (adsorbed) → NH3 (g)
|
|
Reaction 5 actually consists of three steps, forming NH, NH
2,
and then NH
3. Experimental evidence suggests that reaction 2 is
the slow, rate-determining step. This is not unexpected given that the bond
broken, the nitrogen triple bond, is the strongest of the bonds that must
be broken.
Return to the
course outline
or move on to Lecture 4:
Oxygen and its compounds.
References
Much of the information in these course notes has been sourced from Wikipedia under
the Creative Commons License.
DNA modelling
'Inorganic Chemistry' - C. Housecroft and A.G. Sharpe, Prentice Hall, 4th Ed.,
2012, ISBN13: 978-0273742753, pps 24-27, 43-50, 172-176, 552-558, 299-301, 207-212
'Basic Inorganic Chemistry' - F.A. Cotton, G. Wilkinson and P.L.
Gaus, John Wiley and Sons, Inc. 3rd Ed., 1994.
'Introduction to Modern Inorganic Chemistry' - K.M. Mackay, R.A.
Mackay and W. Henderson, International Textbook Company, 5th Ed., 1996.

This work is licensed under a Creative Commons
Attribution-ShareAlike 3.0 Unported License.
 Return to Chemistry,
UWI-Mona, Home Page
Created and maintained by Prof. Robert J.
Lancashire,
Return to Chemistry,
UWI-Mona, Home Page
Created and maintained by Prof. Robert J.
Lancashire,
The Department of Chemistry, University of the West Indies,
Mona Campus, Kingston 7, Jamaica
Created November 2014. Links checked and/or last
modified 17th February 2015.
URL
http://wwwchem.uwimona.edu.jm/courses/CHEM1902/IC10K_MG_hydrogen.html

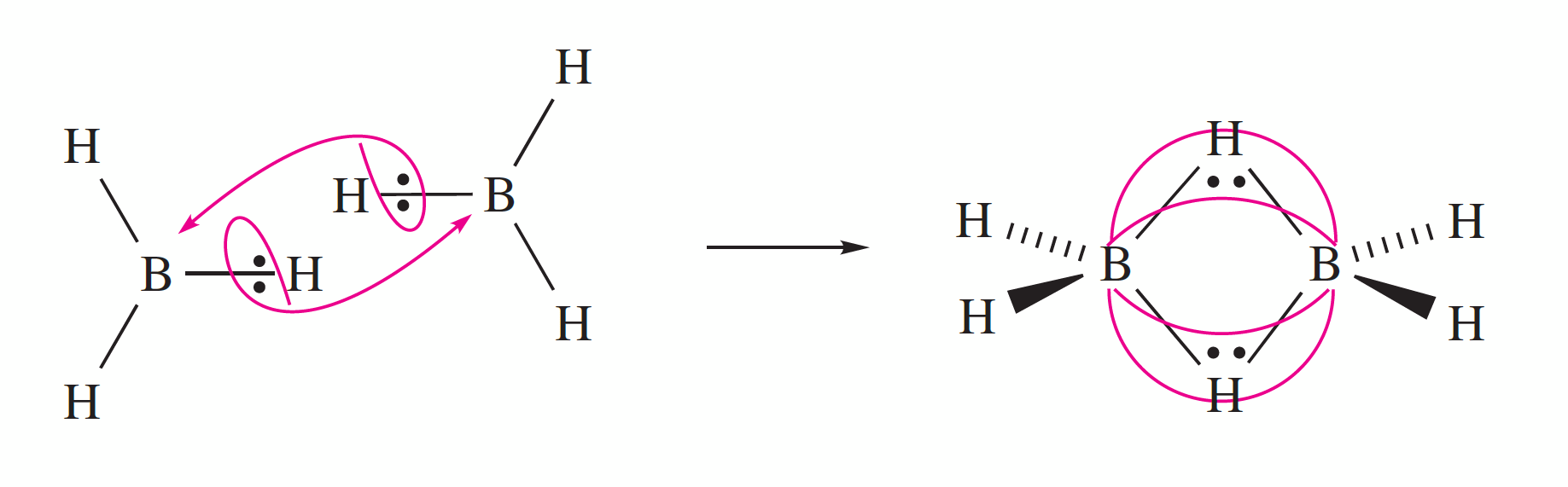




 Return to Chemistry,
UWI-Mona, Home Page
Created and maintained by Prof. Robert J.
Lancashire,
Return to Chemistry,
UWI-Mona, Home Page
Created and maintained by Prof. Robert J.
Lancashire,