Some chemistry of Iron
An excellent site for finding the properties of the elements,
including iron is at 
For the chapter on Iron chemistry from the Elsevier text
"Chemistry of the Elements" by Greenwood and Earnshaw see
On-Line Metals Based Surveys
History
For some background information on the origin and history of Iron
see the Rossell Forge Site in Andorra
Introduction
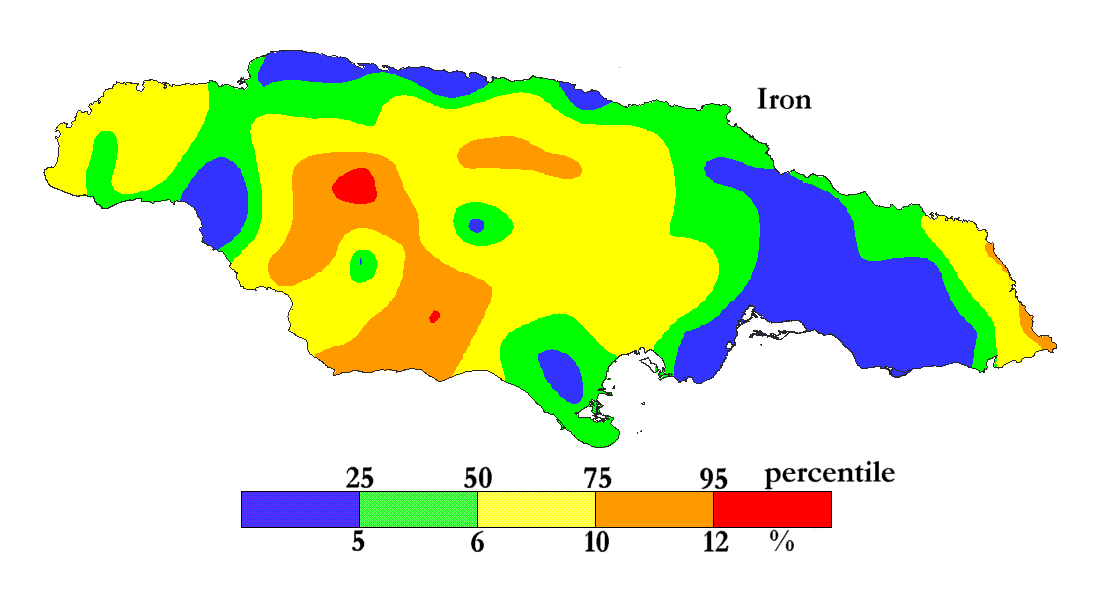
Iron is the most abundant transition metal on Earth (62000 ppm).
The International Centre for Environmental and Nuclear Sciences (ICENS)
has an on-going programme of mapping the geochemical content of Jamaica.
'A Geochemical Atlas of Jamaica' was published in 1995 and is available from Amazon or ICENS.
The results found for Iron are shown below (courtesy of Prof G.C. Lalor).
Extraction of Iron
Iron is generally extracted in a Blast
furnace.
Iron Halides
Iron(III) halides
| Formula |
Colour |
MP |
Structure |
| FeF3 |
green |
1000 sublimes |
| FeCl3 |
black |
306 sublimes |
BiI3 |
| FeBr3 |
dark-red-brown |
decomposes above 200°C |
BiI3 |
Preparations:
Prepared by reaction of Fe + X2 →
FeX3.
Note that FeBr3.aq when boiled gives
FeBr2.
An important application of the chloride is as an
etching material for copper electrical printed circuits.
Iron(II) halides
| Formula |
Colour |
MP |
BP |
Structure |
| FeF2 |
white |
1000 |
1100 |
rutile |
| FeCl2 |
pale yellow-grey |
670-674 |
- |
CdCl2 |
| FeBr2 |
yellow-green |
684 |
- |
CdI2 |
| FeI2 |
grey |
red heat |
- |
CdI2 |
Preparations:
Fe +HX at red heat → FeX2 for X=F,Cl and
Br
Fe + I2 → FeI2
Iron Oxides and Aqueous Chemistry
Iron oxides
| Formula |
Colour |
Oxidation State |
MP |
Structure / comments |
| Fe2O3 |
red brown |
Fe3+ |
1560d |
α-form Haematite,
β-form used in cassettes |
| Fe3O4 |
black |
Fe2+/3+ |
1538d |
magnetite/lodestone |
| FeO |
black |
Fe2+ |
1380 |
pyrophoric |
Preparations:
α-Fe2O3 is obtained
by heating alkaline solutions of Fe(III) and dehydrating the
solid formed.
FeO,Fe3O4, γ-Fe2O3 ccp
α-Fe2O3 hcp
The Fe(III) ion is strongly acidic:
[Fe(H2O)6]3+ + H2O → [Fe(H2O)5(OH)]2+ + H3O+ K=10-3.05
[Fe(OH)(H2O)5]2+ + H2O → [Fe(OH)2(H2O)4]+ + H3O+ K=10-3.26
olation
2Fe(H2O)63+ + 2H2O → [Fe2(OH)2(H2O)8]4++ 2H3O+ K=10-2.91
The Fe2+ ion is barely acidic:
Fe(H2O)62+ + H2O → [Fe(OH)(H2O)5]+ + H3O+ K=10-9.5
Rusting of Iron
The economic importance of rusting is such that it has been
estimated that the cost of corrosion is over 1% of the world's
economy. (25% of the annual steel production in the USA goes
towards replacement of material that has corroded.)
Rusting of iron consists of the formation of hydrated oxide,
Fe(OH)3 or FeO(OH), and is an electrochemical process
which requires the presence of water, oxygen and an electrolyte -
in the absence of any one of these rusting does not occur to any
significant extent. In air, a relative humidity of over 50%
provides the necessary amount of water and at 80% corrosion is
severe.
The process is complex and will depend in detail on the
prevailing conditions, for example, in the presence of a small
amount of O2
the anodic oxidation will be: Fe → Fe2+ +
2e-
and the cathodic reduction: 2H2O + 2e-
→ H2 + 2OH-
i.e. overall: Fe + 2H2O → H2 +
Fe2+ + 2OH-
i.e Fe(OH)2 and this precipitates to form a coating that slows further corrosion.
If both water and air are present, then the corrosion can be
severe with oxygen now as the oxidant
the anodic oxidations: 2Fe → 2Fe2+ +
4e-
and the cathodic reduction: O2 + 2H2O +
4e- → 4OH-
i.e. overall: 2Fe + O2 + 2H2O →
2Fe(OH)2 with limited O2, magnetite is
formed (Fe3O4), otherwise the familiar
red-brown
Fe2O3 H2O “rust” is
found.
The presence of an electrolyte is required to provide a pathway
for the current and, in urban areas, this is commonly iron(II)
sulfate formed as a result of attack by atmospheric
SO2 but, in seaside areas, airborne particles of salt
are important.
The anodic oxidation of the iron is usually localized in surface
pits and crevices which allow the formation of adherent rust over
the remaining surface area.
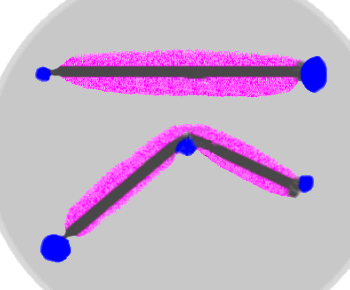
The illustration above shows 2 nails immersed in an agar gel
containing phenolphthalein and [Fe(CN)6]3-.
The nails can be seen to have started to corrode since the
Prussian blue formation indicates the formation of Fe(II) (the Anodic
sites which correspond to the end of the nails and the bend in the middle).
The phenolphthalein (change to pink in presence of base)
shows the build up of OH- and shows that essentially
the whole length of the nail is acting as the cathode.
Eventually the lateral extension of the anodic area undermines
the rust to produce loose flakes. Moreover, once an adherent film
of rust has formed, simply painting over gives but poor
protection. This is due to the presence of electrolytes such as
iron(II) sulfate in the film so that painting merely seals in the
ingredients for anodic oxidation. It then only requires the
exposure of some other portion of the surface, where cathodic
reduction can take place, for rusting beneath the paint to
occur.
The protection of iron and steel against rusting takes many
forms, including: simple covering with paint; coating with
another metal such as zinc (galvanizing) or tin; treating with
"inhibitors" such as chromate(VI) or (in the presence
of air) phosphate or hydroxide, all of which produce a coherent
protective film of Fe2O3. Another method
uses sacrificial anodes, most usually Mg or Zn which, being
higher than Fe in the electrochemical series, are attacked
preferentially. In fact, the Zn coating on galvanized iron is
actually a sacrificial anode.
Rust prevention
Galvanised iron is the name given to iron that has been dipped
into molten zinc (at about 450°C) to form a thin covering of zinc
oxide. One level of rust prevention occurs through a purely
mechanical method since it is more difficult for water and oxygen
to reach the iron. Even if the layer becomes somewhat worn though
another reason corrosion is inhibited is that the anodic
processes are affected.
The E° for zinc oxidation (0.76V) is considerably more positive
that E° for iron oxidation (0.44V) so the zinc metal is oxidized
before the iron. Zn2+ is lost to the solution and the
zinc coating is called a sacrificial
anode.
Foodstuffs are often distributed in "tin cans" and it
has generally been easier to coat the iron with a layer of tin
than with zinc. Another benefit is that tin is less reactive then
zinc so does not react as readily with the contents. However the
electrode oxidation potential for Sn/Sn2+ is 0.14V so
once again iron becomes the anode and rust will occur once the
coating is worn or punctured.
Another technique is to treat the iron surface with dichromate
solution.
2 Fe + 2 Na2CrO4 + 2 H2O
-> Fe2O3 + Cr2O3 + 4
NaOH
The iron oxide coating formed has been found to be impervious to
water and oxygen so no further corrosion can occur.
The FeIII/FeII Couples
A selection of standard reduction potentials for some iron
couples is given below, from which the importance of the
participating ligand can be judged. Thus Fe(III), being more
highly charged than Fe(II) is stabilized (relatively) by
negatively charged ligands such as the anions of edta and
derivatives of 8-hydroxyquinoline, whereas Fe(II) is favoured by
neutral ligands which permit some charge delocalization in
π-orbitals (e.g. bipy and phen).
Table E° at 25°C for some FeIII/FeII couples in
acid solution
| FeIII |
|
FeII |
E°/V |
| [Fe(phen)3]3+ |
+ e- → |
[Fe(phen)3]2+ |
1.12 |
| [Fe(bipy)3]3+ |
+ e- → |
[Fe(bipy)3]2+ |
0.96 |
| [Fe(H2O)6]3+ |
+ e- → |
[Fe(H2O)6]2+ |
0.77 |
| [Fe(CN)6]3- |
+ e- → |
[Fe(CN)6]4- |
0.36 |
| [Fe(C2O4)3]3- |
+ e- → |
[Fe(C2O4)2]2- +
(C2O4)2- |
0.02 |
| [Fe(edta)]- |
+ e- → |
[Fe(edta)]2- |
-0.12 |
| [Fe(quin)3] |
+ e- → |
[Fe(quin)2] + quin- |
-0.30 |
where quin- = 5-methyl-8-hydroxyquinolinate,
The value of E° for the couple involving the simple aquated ions,
shows that Fe(II)(aq) is thermodynamically stable with respect to
hydrogen; which is to say that Fe(III)(aq) is spontaneously
reduced by hydrogen gas. However, under normal circumstances, it
is not hydrogen but atmospheric oxygen which is important and,
for the process 1/2O2 + 2H+ +
2e- → H2O, E° = 1:229 V, i.e. oxygen gas is
sufficiently strong an oxidizing agent to render
[Fe(H2O)6]2+ (and, indeed, all
other Fe(II) species in the Table) unstable with respect to
atmospheric oxidation. In practice the oxidation in acidic
solutions is slow and, if the pH is increased, the potential for
the Fe(III)/Fe(II) couple remains fairly constant until the
solution becomes alkaline and hydrous Fe2O3
(considered here for convenience to be Fe(OH)3) is
precipitated. But here the change is dramatic, as explained
below.
The Redox chemistry of Iron is pH dependent:
Fe(H2O)63+ + e- → Fe(H2O)62+
E°=0.771V
The actual potential E of the couple is given by the Nernst
equation,
where E= E° when all activities are unity. However, once
precipitation occurs, the activities of the iron species are far
from unity; they are determined by the solubility products of the
2 hydroxides. These are:
[Fe(III)][OH-]2 ~ 10-14 (mol
dm-3)3 and
[Fe(III)][OH-]3 ~ 10-36 (mol
dm-3)4
Therefore when [OH-] =1 mol dm-3;
[FeII]/[FeIII] ~ 1022
Hence E ~ 0.771 - 0.05916 log10.(1022) =
0.771 - 1.301 = -0.530 V
Thus by making the solution alkaline the sign of E has been
reversed and the susceptibility of Fe(II)(aq) to oxidation (i.e.
its reducing power) enormously increased. In base the white,
precipitated Fe(OH)2 and FeCO3 is a good reducing agent
and samples are rapidly darkened by aerial oxidation and
this explains why Fe(II) in alkaline solution
will reduce nitrates to ammonia and copper(II) salts to metallic
copper.
Representative complexes
An important Fe complex which is used in Actinometry since it
is photosensitive is K3[Fe(C2
O4)3.3H2O.
It can be prepared from:
Fe(C2O4) in
K2C2O4 by reacting with
H2O2 in
H2C2O4 to give green crystals.
It is high spin μ =5.9 BM at 300K and has
been resolved into its two optical isomers, although they
racemise in less than 1 hour.
In light the reaction is:
K3Fe(C2O4)3.3H2O → 2Fe(C2O4) + 2CO2 + 3K2C2O4
Another important complex is used as a redox indicator
since the Fe(II) and Fe(III) complexes are both quite stable and
have different colours:
Fe(phen)33+ + e- → Fe(phen)32+ E°=1.12V
blue red
The ligand is 1,10 phenanthroline and the indicator is called ferroin.
An interesting example of how acetates can bind to metal ions is seen in what
has been described as a "molecular ferric wheel".
The structure was determined in 1990 and contains [Fe(OMe)2(O2CCH2Cl)]10
see J. Amer. Chem. Soc., 1990, 112, 9629.
|
References:
"Complexes and First-Row Transition Elements", D. Nicholls
"Basic Inorganic Chemistry", F.A. Cotton, G. Wilkinson and P.L.
Gaus
"Advanced Inorganic Chemistry", F.A. Cotton, G. Wilkinson, C. A.
Murillo, and M. Bochmann
"Chemistry of the Elements", Greenwood and Earnshaw
return to the C21J course
outline
 Return to Chemistry, UWI-Mona,
Home Page
Return to Chemistry, UWI-Mona,
Home Page
Copyright © 2001-2008 by Robert John Lancashire,
all rights reserved.
Created and maintained by Prof. Robert J.
Lancashire,
The Department of Chemistry, University of the West Indies,
Mona Campus, Kingston 7, Jamaica.
Created October 2001. Links checked and/or last
modified 12th November 2008.
URL
http://wwwchem.uwimona.edu.jm/courses/iron.html

 Return to Chemistry, UWI-Mona,
Home Page
Return to Chemistry, UWI-Mona,
Home Page