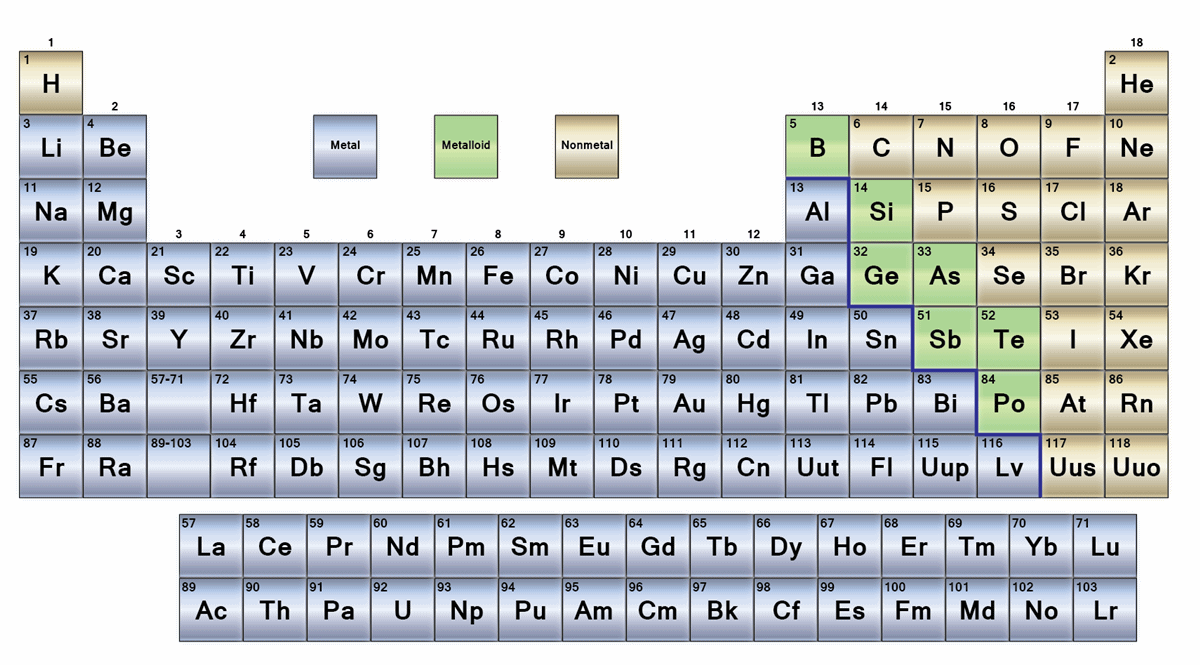

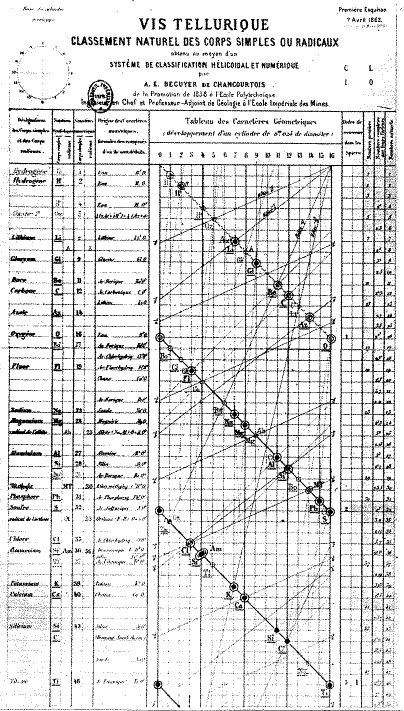
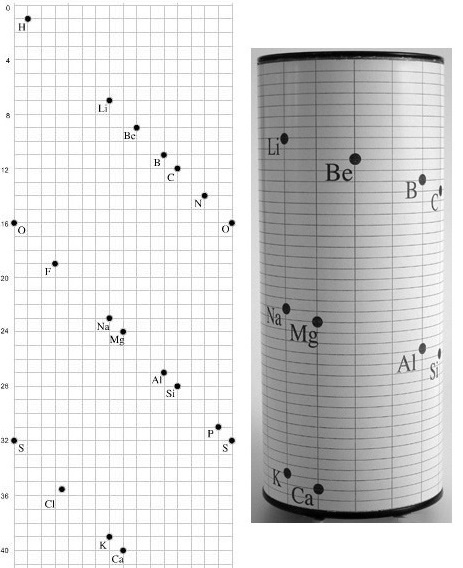

| Group number | Recommended name |
|---|---|
| 1 | Alkali metals |
| 2 | Alkaline earth metals |
| 15 | Pnictogens |
| 16 | Chalcogens |
| 17 | Halogens |
| 18 | Noble gases |
| Charles Janet Periodic Table (~1930) | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| f1 | f2 | f3 | f4 | f5 | f6 | f7 | f8 | f9 | f10 | f11 | f12 | f13 | f14 | d1 | d2 | d3 | d4 | d5 | d6 | d7 | d8 | d9 | d10 | p1 | p2 | p3 | p4 | p5 | p6 | s1 | s2 | |
| 1 H |
2 He |
|||||||||||||||||||||||||||||||
| 1 H |
2 He |
3 Li |
4 Be |
|||||||||||||||||||||||||||||
| 5 B |
6 C |
7 N |
8 O |
9 F |
10 Ne |
11 Na |
12 Mg |
|||||||||||||||||||||||||
| 13 Al |
14 Si |
15 P |
16 S |
17 Cl |
18 Ar |
19 K |
20 Ca |
|||||||||||||||||||||||||
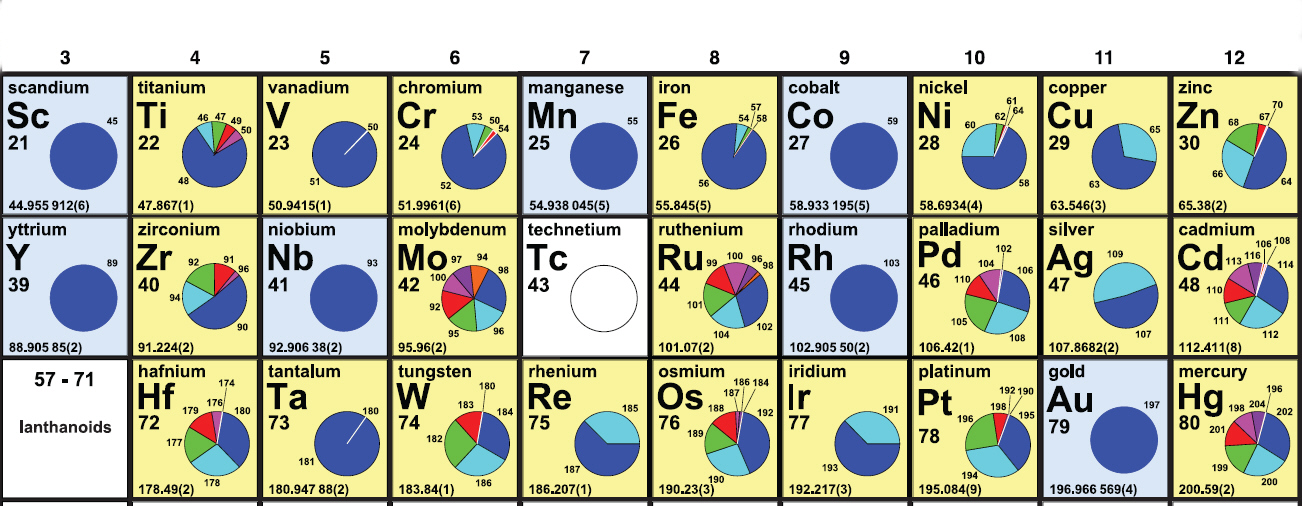
| 21 Sc |
22 Ti |
23 V |
24 Cr |
25 Mn |
26 Fe |
27 Co |
28 Ni |
29 Cu |
30 Zn |
31 Ga |
32 Ge |
33 As |
34 Se |
35 Br |
36 Kr |
37 Rb |
38 Sr |
|||||||||||||||
| 39 Y |
40 Zr |
41 Nb |
42 Mo |
43 Tc |
44 Ru |
45 Rh |
46 Pd |
47 Ag |
48 Cd |
49 In |
50 Sn |
51 Sb |
52 Te |
53 I |
54 Xe |
55 Cs |
56 Ba |
|||||||||||||||
| 57 La |
58 Ce |
59 Pr |
60 Nd |
61 Pm |
62 Sm |
63 Eu |
64 Gd |
65 Tb |
66 Dy |
67 Ho |
68 Er |
69 Tm |
70 Yb |
71 Lu |
72 Hf |
73 Ta |
74 W |
75 Re |
76 Os |
77 Ir |
78 Pt |
79 Au |
80 Hg |
81 Tl |
82 Pb |
83 Bi |
84 Po |
85 At |
86 Rn |
87 Fr |
88 Ra |
|
| 89 Ac |
90 Th |
91 Pa |
92 U |
93 Np |
94 Pu |
95 Am |
96 Cm |
97 Bk |
98 Cf |
99 Es |
100 Fm |
101 Md |
102 No |
103 Lr |
104 Rf |
105 Db |
106 Sg |
107 Bh |
108 Hs |
109 Mt |
110 Ds |
111 Rg |
112 Cn |
113 Uut |
114 Fl |
115 Uup |
116 Lv |
117 Uus |
118 Uuo |
119 Uue |
120 Ubn |
|
|
|
||||||||||||||||||||||||||||||||
| Particle | Charge | Mass (g) | Mass (amu) |
|---|---|---|---|
| Proton | +1 | 1.6726 x 10-24 g | 1.0072766 |
| Neutron | 0 | 1.6749 x 10-24 g | 1.0086654 |
| Electron | -1 | 9.110 x 10-28 g | 0.000548597 |
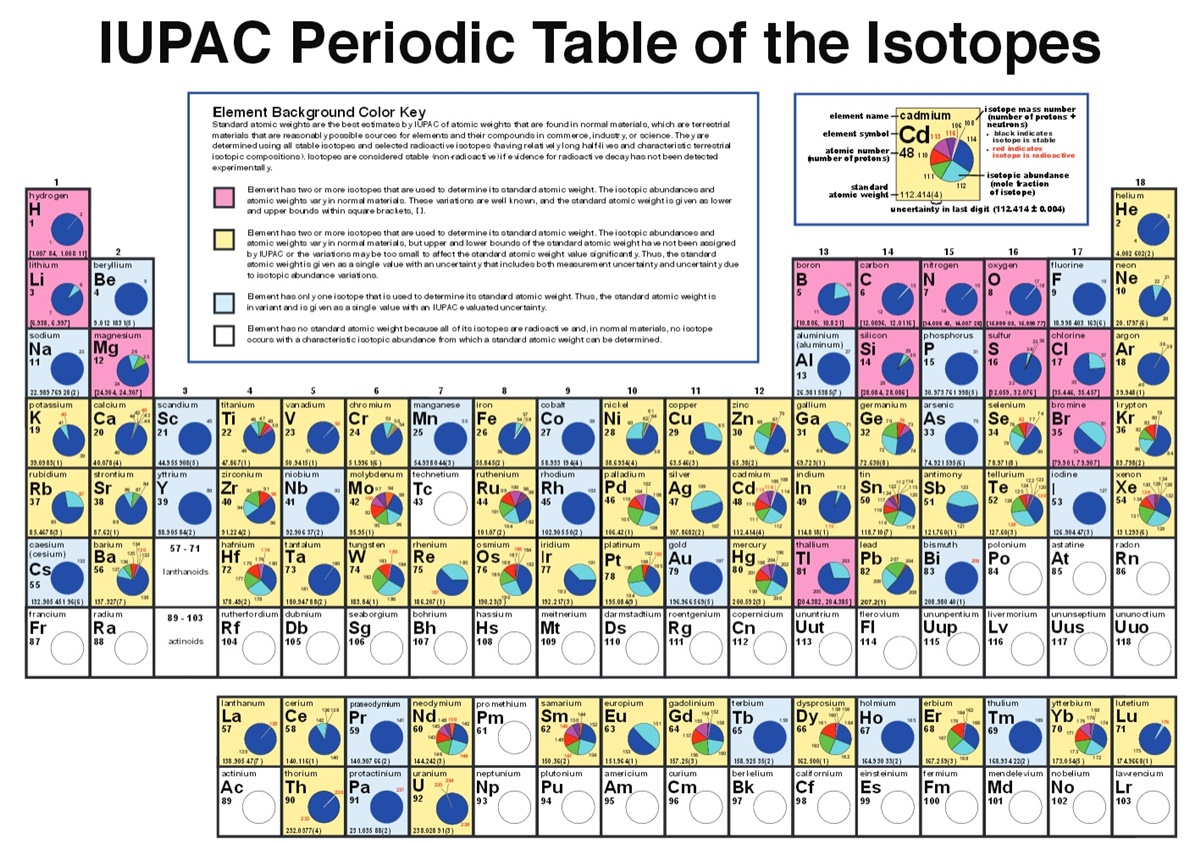
 Return to Chemistry, UWI-Mona,
Home Page
Return to Chemistry, UWI-Mona,
Home Page
Copyright © 2004-2015 by Robert John Lancashire, all rights reserved.
Created and maintained by Prof. Robert J. Lancashire,